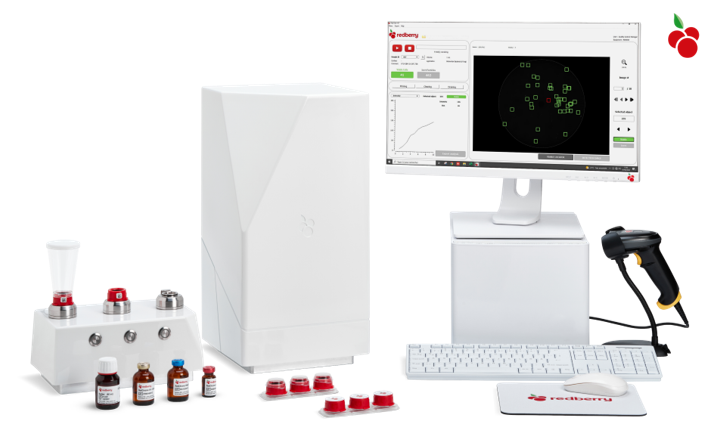
Red One™ is an automated platform for rapid and quantitative microbiological detection.
A 2-step sterility testing method has been developed in accordance with USP <71> and Ph. Eur. 2.6.1.
I. Enrichment phase in compendial liquid media (TSB & FTM)- Filtrable products: strictly based on sample preparation in standard double-canister device
- Non-filtrable products: direct inoculation in bottles
- Incubation: 4 days for LOD= 1 CFU (Ph. Eur. 2.6.1) or 24h for LOD < 100 CFU (Ph. Eur. 2.6.27)
- Sampling 1mL from each canister
- Dropping onto RedCaps (touch-free membrane)
- Analysis on Red One™: 10 minutes per RedCap
For cellular matrices, a cell separation step is added before analysis on Red One™.
How does it work?Based on the new generation of solid phase cytometry technology, Red One™ detects viable cells by using advanced image processing techniques to track and analyze the assimilation of staining agents by the targeted cells (enzymatic labeling).
This 10-minute real-time fluorescence analysis enables a very reliable differentiation of targeted viable cells from inert particles and provides an unprecedented level of sensitivity.
Developed to fulfill your needs:- Easy: Ready to use. No calibration is required. Drop the sample on the cap and close the drawer. Filtration, staining, and analysis are automated.
- Precise: Direct counting of cells in 1ml - after enrichment phase in compendial liquid media
- Rapid: Analysis performed on Red One™ in 10 min. TTR= 4 days for LOD=1 CFU.
- Post-analysis identification: In case of a positive result, identification with traditional methods remains possible.
Discover our 10-min analysis with Red One™
Red One™ fits perfectly in a working environment - production or laboratory - including under Laminar Flood Hood.
Water and surface environmental rapid testing solutions have been developed on the same Red One™ platform to complete your contamination control strategy.
Do you intend to implement a sterility testing method for your medicinal products? Learn more or contact us using the green "Request Information" button below, and we’ll be happy to tell you more and listen to your needs!