Material transfer remains one of the most vulnerable steps in sterile manufacturing, and a key focus of the revised EU GMP Annex 1. As the guidance notes, the movement of equipment and materials into and out of cleanrooms and critical zones is “one of the greatest potential sources of contamination.” It’s also a common area of regulatory non-compliance, particularly where packaging protocols are underdeveloped or inconsistently applied.
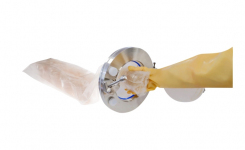
Many facilities are therefore turning to packaging formats that support safer, staged transfer of materials into higher-grade cleanroom environments. Solutions such as AnalytiChem’s Redipor® TwistLock plates and BetaBag exemplify how validated systems can help maintain sterility during handling and transportation. By reducing manual intervention and enabling compatibility with isolators and RABS, these packaging formats support aseptic workflows and alignment with Annex 1 expectations.
Key packaging considerations under Annex 1:
- Triple- and double-wrap formats enable staged transfer through cleanroom zones
- Locking plates such as TwistLock help maintain sample integrity, reducing the risk of accidental exposure and false positives.
- Validated packaging supports disinfection procedures and limits manual handling.
- Packaging must be robust, sealed, and suitable for RABS or isolator us.e
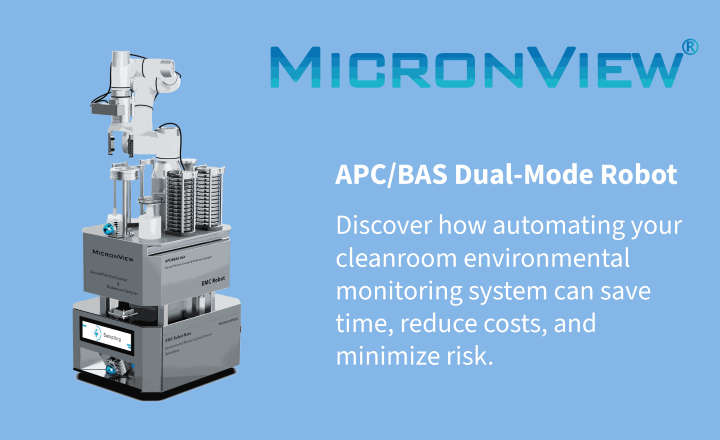
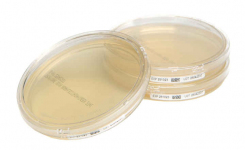
Environmental monitoring is another critical pillar of Annex 1 compliance. SAS air samplers, available from AnalytiChem in Europe, support risk-based, in-process monitoring across cleanroom zones. When paired with validated prepared media, such as Redipor formulations, these tools help ensure consistent microbial control and strengthen your overall contamination control strategy.
Together, robust packaging, validated media, and reliable monitoring tools form a critical foundation for sterility assurance. As Annex 1 places greater emphasis on contamination control and risk-based strategies, integrating these elements into a cohesive approach is key to achieving and maintaining compliance.