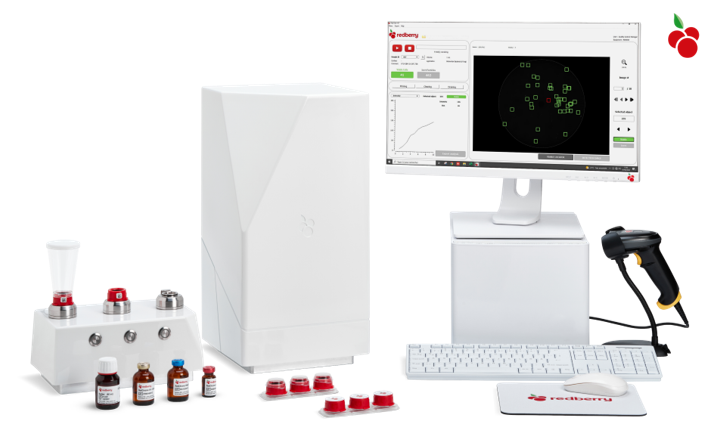
By leveraging single-cell detection, Red One™ delivers faster results compared to traditional microbiology methods, enabling quicker confirmation of pharmaceutical product quality and expedited product release.
How Does It Work?
Red One™, based on cutting-edge Solid Phase Cytometry technology, utilizes advanced image processing to detect viable cells by monitoring the assimilation of staining agents through enzymatic labeling. With its 10-minute real-time fluorescence analysis, it offers highly reliable differentiation of viable cells from inert particles, achieving unmatched sensitivity and speed. Red One™ operates with a high level of automation, eliminating the need for human interpretation, thereby enhancing analysis repeatability and robustness.
Versatile Applications Across Pharmaceuticals and Healthcare
Red One™ is compatible with a broad spectrum of filtrable samples found within the pharmaceutical, biotech, and personal care industries, including raw materials, in-process water (such as purified water and WFI), sterile final products (including biological matrices, radiopharmaceuticals and traditional drugs), non-sterile final products (especially aqueous preparations), and cell-based products, including cell and gene therapies (after pre-treatment.
Bioburden testing is a pivotal quality control step for contamination detection, in accordance with Ph. Eu. 2.6.12 and USP <62> guidelines. Traditional compendial methods rely on microbial growth, which may not align with manufacturers' demands for swifter operations. Red One™ offers a rapid detection solution based on the activation of total aerobic flora, providing both speed and accuracy.
All three bioburden testing applications follow the same streamlined 3-step workflow shown in this video:
Three distinct applications are available:
BB – Bacteria Including Spores: Enumeration of aerobic total bacterial flora, Time-to-Result (TTR) = 3 hours, Quantitative result - Limit Of Quantification (LOQ) = 5 viable cells per tested sample
BB – Bacteria Fungi: Enumeration of aerobic total mesophilic flora Time-to-Result (TTR) = 4 hours Quantitative result - LOQ= 5 viable cells per tested sample
BB – 0 CFU: Presence/absence assay, with a 24-hour enrichment phase, allowing sufficient growth to detect the presence of even very low inoculum. Time-to-Result (TTR) = 24 hours Qualitative result - Limit of Detection (LOD) = 1 viable cell per tested sample Ideal for situations where 0 CFU is expected, such as for bulk drug substances release.
The three-step workflow involves:- Filtration of both sample and activation/enrichment buffer
- Activation/enrichment phase (3 to 24h incubation)
- Automated analysis on Red One™
Accelerated Sterility Release Testing in 4 Days
Compendial sterility testing takes 14 days for a final result, as per pharmaceutical industry standards (Ph. Eu. 2.6.1 and USP <71>). Red One accelerates this timeline to achieve results in just 4 days while keeping the standard sample preparation protocol (and the possibility of post-identification) thanks to its 2-step sterility testing:
Step 1. Enrichment phase in compendial liquid media (TSB & FTM)
- Filtrable products: strictly based on sample preparation in standard double-canister device
- Non-filtrable products: direct inoculation in bottles
- Incubation: 4 days for LOD= 1 CFU (Ph. Eur. 2.6.1) or 24h for LOD < 100 CFU (Ph. Eur. 2.6.27)
Step 2. Analysis performed on Red One™
- Sampling 1mL from each canister
- Dropping onto a Red Cap
- Analysis on Red One™: 10 minutes per Red Cap
- For cellular matrices, a cell separation step based on a selective lysis is applied before the analysis
No need to change your sample preparation - Red One™ offers a quicker read-out than any other method.
In Summary

Stay Compliant and Informed
Red One™ software suite complies with 21 CFR part 11 requirements, ensuring data integrity and regulatory compliance. The Redberry team provides comprehensive support for equipment roll-out in GMP environments.
Join us at the Pharmalab Congress to witness the presentation of preliminary validation results for rapid sterility testing!
Find out more, or use the green "Request Information" button below to ask for details.