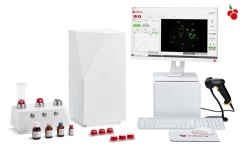
Redberry has announced the commercial launch of a fully automated rapid microbial detection platform for in-process control and industrial products release.
Unprecedented performances for quantification of microbial load of waters and pharmaceutical products in ten minutes.
When present in finished products, bacteria, yeasts and molds may create a health hazard or product spoilage, possibly leading to product recalls and a loss of brand reputation. Standard laboratory results can take several days to be reported, resulting in either lost production or delayed product release of water, food, cosmetics and pharmaceutical products.
Fully automated, Red One™ is an innovative, rapid and very easy-to-use solution to detect the presence of micro-organisms in raw materials or finished products. No calibration is required, the sample is placed onto a proprietary single-use capsule and automatically filtered then analysed using the unique Red One™ detection platform. Red One™ detects single viable cells by using advanced image processing techniques. The patented system tracks and analyzes the assimilation of staining agents by the targeted cells (“staining kinetics”). The analysis is performed in real-time and enables differentiation of targeted viable cells from background debris (typically inert particles) with a very high level of reliability.
Red One™ performs analysis in a single-step workflow. "Tests can then be performed not only in a laboratory but also close to where the samples are taken. On the production line, it enables real-time control and monitoring," explains Jonathan Macron, CEO of Redberry.
Delivering results in just ten minutes, Red One™ can provide actionable data, highlighting contamination issues as they occur so timely remedial action can be taken and product wastage reduced.
"Detection of microorganisms traditionally relies on culture methods which provide results after a few days of incubation. Rapid alternative methods such as ATP monitoring or flow cytometry may provide results in a few tens of minutes. But they often correlate poorly to culture-based methods and their limit of detection (typically 100 to 1000 CFU/mL without enrichment) is not sensitive enough to accurately assess the level of bioburden that is required for waters or pharmaceutical products. Based on numerous benchmarks from customers, Red One™ showed unprecedented results in terms of sensitivity and repeatability," says Dr. Joseph Pierquin, Founder & CTO of Redberry.
Red One™ software suite ensures data integrity compliance according to 21 CFR Part 11: fully searchable audit trail, user control and management, and reliable data generation and backup.
Leveraging on its core technology, Redberry aims at delivering the fastest solution for rapid sterility testing of vaccines and drugs in 2021.
Pharma companies are now racing to sufficient supplies of vaccine for Coronavirus SARS-CoV-2 (COVID-19) to meet global demand. The need for rapid testing to ensure vaccines are safe before they can be released to patients is therefore critical.
The current culture-based method requires a 14-day sterility test which, in the current pandemic emergency situation, is too long. The need to dramatically reduce this Time-To-Result (TTR) requires new approaches, not only for vaccines but also for short shelf-life sterile drugs such as Advanced Therapy Medicinal Products (ATMP).
Redberry has started to validate the workflows associated with using its platform for release with its early adopters. Major pharma companies and an independent contract service laboratory will collaborate with Redberry to confirm that RedOne™ could assess the sterility of pharmaceutical products in most cases within 48 hours. This will position the platform as the fastest method to detect down to one single culturable micro-organism in comparison with current alternative rapid methods claiming a 5-day TTR.
Roll-out in 2021 will involve forming a strategic alliance with a global market leader leveraging their existing framework and expertise to reach this target market. "We think partnering with an established company is key for our success in the pharmaceutical sector. Discussions are already ongoing with several potential partners," mentions Macron.
More products are in development, for specific detection and in-line control.
"We develop step by step. Our first objective was to confirm the added-value of the microbiological performances of our core technology. This has now been successfully achieved with our early adopters all confirming their wish to order a platform after evaluating it. We are proud to officially launch our first product today and to start delivery thisweek. The team is now working on a range of solutions for the detection of specific organisms and in-line control. The best is yet to come," concludes Macron.
The new Red One™ platform, which is available now in France, Germany & Switzerland will be launched in other European countries and North America in the coming months.
For more information visit www.redberry.net