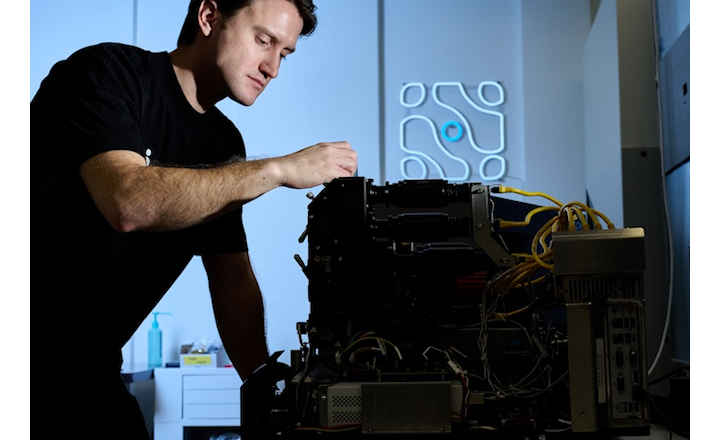
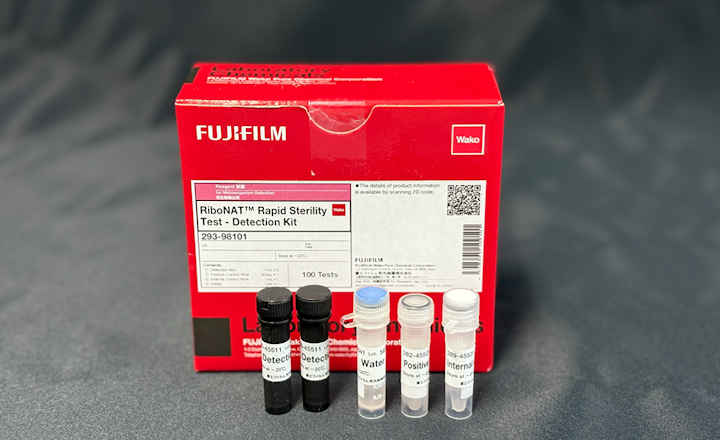
Redberry introduces a breakthrough in sterility testing: a fully validated rapid method delivering results in less than 4 days using the Red One™ platform. Designed for pharmaceutical manufacturers seeking to accelerate batch release without compromising compliance or accuracy, this solution leverages automated solid-phase cytometry. It provides a faster read-out while maintaining unchanged sample preparation (same media, containers, incubation conditions). This preparation allows both rapid read-out in <4 days and compendial read-out at 14 days.
Why choose Red One™ for sterility testing?
- Validated according to Ph. Eur. 5.1.6 & USP <1223>: full primary validation package available now
- Results in <4 days: faster batch release, better agility
- Fully automated: no manual handling, no microscope, no subjective interpretation
- Wide matrices compatibility including cell-based, radiopharmaceuticals and mRNA vaccines
- Unchanged sample preparation: rapid read-out <4 days and compendial read-out at 14 days
- Straightforward identification: compatible with usual methods (MALDI-TOF, sequencing, etc.)
- Rapid implementation: full support for streamlined product-specific validation
What’s next?
Our team of experts will guide you through the product-specific validation process, enabling adoption of Red One™ with confidence and minimal delay. Several pharmaceutical manufacturers have already accelerated their workflows thanks to this supported validation path.
Looking ahead, Redberry is developing a next-generation sterility test delivering results in under 48 hours, designed for products with a very short shelf life and sample volumes up to 1 mL.
Meet us at upcoming events
Come meet us at the PDA Microbiology Conference in Washington DC (USA, October 2025) - Booth 406 or at the Pharmalab Conference in Düsseldorf (Germany, November 2025) - Booth 10.