Pharmamedia Dr. Müller (PMM) is a leading manufacturer of culture media for environmental monitoring (EM) and is specialized in the production as well as the development of microbiological media for pharmaceutical applications. Triggered by customer requests, PMM continuously expands its product portfolio and provides innovative products with a high level of quality and safety.
Environmental monitoring processes in pharmaceutical industries are constantly evolving and PMM uses its many years of experience to always optimize its products, thereby keeping up with this evolution and being able to respond to change demands from customers.
The request for automation in the evaluation of EM plates has been increasingly frequent during the last years. The necessity to convert the evaluation of EM samples from a manual to an automated process is driven by several factors including shortage of personnel, increasing requests from the authorities for
4-eyes-evaluation, necessity for documentation of the evaluation and reduction of time to result.
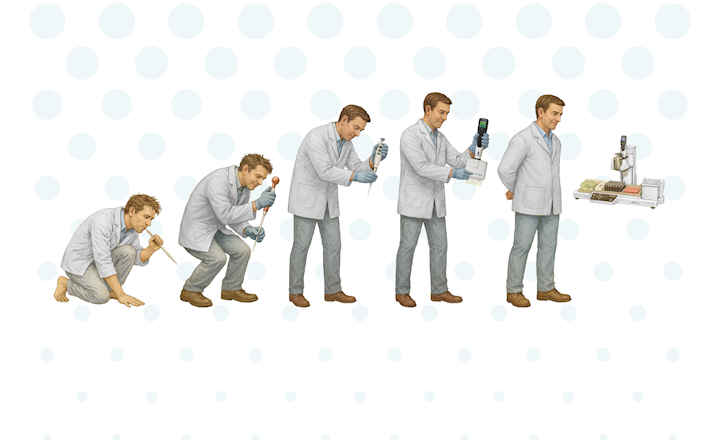
The transition from a manual, subjective approach to a more objective and automated evaluation of environmental monitoring media is a complex and gradual process that progresses steadily through multiple, not necessarily sequential, steps.
Steps include:
- Electronic documentation of plate evaluation only
- Electronic documentation and automatic allocation of the evaluation to the sample
- Electronic plate evaluation (zero vs. non-zero result)
- Electronic colony detection and enumeration
- Automatic evaluation and/or colony count of multiple plates at the end of the incubation period
- Automatic incubation and evaluation and/or colony count of multiple plates
- Automatic and dynamic evaluation of plates at different timepoints during incubation.
Regardless of the grade of automation and the dynamic of the evaluation, a picture of a plate taken with a camera is the starting point for the assessment of any plate, followed by the detection and eventually count of microorganism colonies using a system that has been designed and trained accordingly. To enable the most reliable colony detection and avoid false positive or negative results, it is crucial to remove structures on petri dishes that may interfere with colony detection.
For this reason, PMM is improving the design of both 90 mm and contact plates.
Improvements are made by:
- Removing any labeling on the outer part of the lid of both plate types
- Moving all structures on the outer part of the lid and the base of 90 mm plates to the outside
- Omitting the letters and numbers on the base of contact plates
Follow Us on LinkedIn