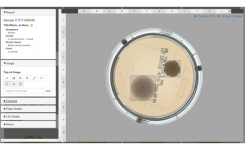
Manual reading and counting of agar plates has evolved over the years resulting in increased regulatory scrutiny throughout the pharmaceutical industry. Specifically, the US FDA has issued 483s concerning the qualification of microbiologists who analyze plates, the lack of proper procedures for handling plates during the plate reads, and the instrument qualification used to perform plate counts.
Although many types of plates are used for various test methods in pharmaceutical microbiology, regulatory actions have focused on environmental monitoring (EM) of classified areas where viable air and surface sampling play an important role in ensuring the safe production of drug products.
Concerns over human errors in plate counts and transcription of results, as well as potential data integrity issues, continue to drive apprehension regarding manual methods amongst regulators calling for a shift from manual processing to automated technologies.
So where do you start?
Join us for a webinar on Tuesday, May 20, 2025 at 10:00AM EST to explore a multi-perspective view on the challenges associated with the manual method of EM plate reading and the ability to implement intelligent automation to improve this process.
Speakers:
- Vanessa Figueroa, Founder & Chief Executive Microbiologist, VVF Science
- Andrew Gravett, Principal Scientist, Microbiology, AstraZeneca
- Jack Brown, Corporate Development Director, Clever Culture Systems
Secure your spot today. Register here.