When it comes to environmental monitoring (EM), manual plate reading isn’t just tedious and inefficient, it’s a risk to your process. Recent 483’s highlight procedural gaps, human error, and data integrity as just some of the issues with the manual method. With a multi-perspective view from three subject matter experts, learn how to overcome these challenges with intelligent automation.
In this on-demand webinar, explore:
- The latest regulatory expectations for EM plate reading
- How to automate plate reading without disrupting your workflow
- Insight from a global pharma company that made the switch
Gain knowledge on this important topic from:
- Vanessa Figueroa - Founder & Chief Executive Microbiologist, pharmaceutical microbiology thought leader
- Andrew Gravett - Principal Scientist of Microbiology at AstraZeneca, who recently implemented APAS Independence–automated EM plate reader powered by artificial intelligence
- Jack Brown - Corporate Development Director at Clever Culture Systems, the company behind APAS technology
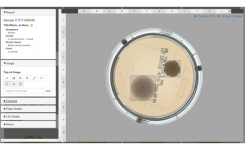
Clever Culture Systems APAS Independence intelligent automation technology provides highly accurate colony differentiation and counting, including automated flagging of any moulds or spreading organisms present on culture plates. The system utilizes a flexible design that does not require proprietary media, allowing for ease of implementation into the EM workflow while reducing manual processes and increasing data integrity.
Building on an established, validated platform, APAS technology is now available to automate the reading of both contact and 90mm plates on a single hardware platform. With the addition of contact plates, APAS Independence is now the only fully automated technology capable of providing high-throughput bulk processing of both contact and 90mm plates used for EM in pharmaceutical manufacturing.
Start your journey to smarter plate reading today, watch the webinar here.