Pharmaceutical manufacturers need both speed and certainty in viable monitoring. Real-time data reveals potential contamination as it happens, but culture confirmation remains required for verification and investigation. MicronView’s Remote BAMS brings these two capabilities together in one compact, intuitive system that has been validated for stability, accuracy, and flexibility in real-world cleanroom environments.
How It Works
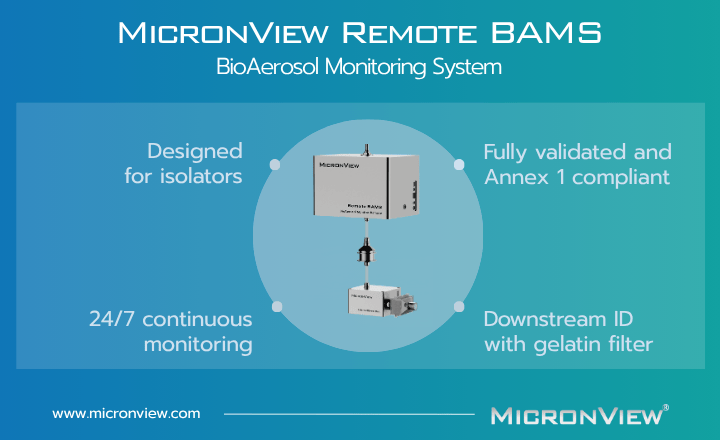
The Remote BAMS integrates biofluorescent particle counting (BFPC) and standard particle monitoring in a single instrument. A 405 nm laser measures total and biofluorescent particles simultaneously, providing instant detection of viable contamination.
An external 37 mm gelatin filter holder collects the same air sample for culture confirmation and microbial identification. Built from 316L stainless steel, the holder can be autoclaved and quickly reinstalled, ensuring aseptic handling and minimal downtime.
The compact, modular design makes installation simple. The Remote BAMS can be installed in isolator systems using an external blower or central vacuum connection, maintaining proper airflow patterns while providing continuous viable and non-viable monitoring.
Validated for Real-world Use
Extensive validation work was performed to confirm that the Remote BAMS, when paired with an external gelatin filter, performs accurately and consistently under conditions representative of pharmaceutical cleanrooms and isolators. The studies were designed to evaluate both long-term sampling stability and the impact of installation geometry on microbial recovery.
- Gelatin Filter Stability: Recovery remained consistent after 18 hours of continuous sampling, proving that the gelatin filter maintains microbial viability during prolonged operation. This is especially critical in Grade A environments where viable events are extremely rare, and monitoring runs can extend across an entire shift or batch. Despite long exposure times, the gelatin membrane did not dry out or lose recovery efficiency, demonstrating its suitability for continuous viable monitoring.
- Tubing Flexibility: The system was tested using multiple tubing configurations, including extended lengths up to 2 meters and bends up to two 45-degree angles. Across all setups, there was no measurable loss in recovery, confirming that the system maintains stable airflow and particle transport even when installed in less direct routing layouts. This flexibility allows users to position the instrument outside isolators or behind enclosures without compromising performance or compliance.
- Stable Internal Controls: In every test, the total particle and biofluorescent particle channels within the Remote BAMS showed highly consistent results. Correlation coefficients approached 1.0 across all runs, confirming that both channels tracked equally throughout the tests. This internal consistency verifies that differences in colony-forming unit (CFU) counts were due to normal biological variability rather than instrument performance or sampling inefficiency.
Together, these studies confirm that MicronView’s Remote BAMS with an external gelatin filter delivers reliable, reproducible performance under realistic operating conditions. Users can confidently rely on the system for both continuous viable monitoring and culture-based confirmation without concerns about extended sampling duration or tubing configuration.
Compliance and Ease of Use
The Remote BAMS supports Annex 1-compliant viable monitoring with both immediate detection and culture confirmation. Users benefit from continuous, traceable data, simple filter replacement, and minimal maintenance. Its small size, intuitive workflow, and validated stability make it an ideal choice for continuous monitoring inside isolators or RABS.