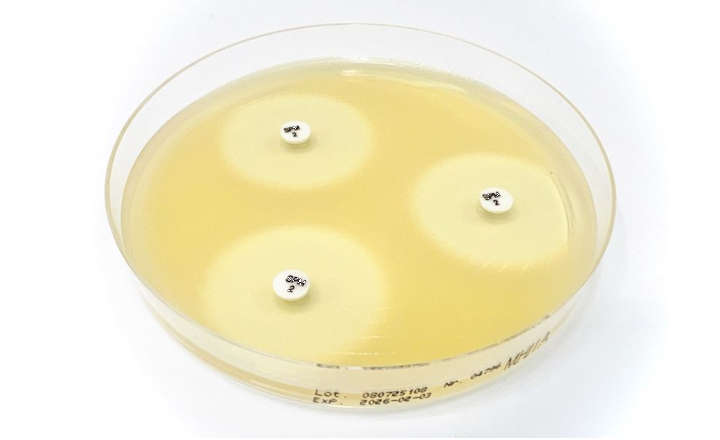
Nissui Pharmaceutical offer the Nissui CompactDry™, a ready to use culture media plate certified by MicroVal (an independent approval and certification organisation) as an alternative method according to ISO16140-2.
Here, Nissui Pharmaceutical introduce a series of on-demand webinars:
- Webinar 1 - Test Method Validation That Can be Trusted
- Webinar 2 - Method Validation and Verification
In these short webinars, Roy Betts, Research Fellow at Campden BRI, UK and Chairman of the MicroVal general committee explores what is the role of MicroVal? What is ISO16140-2? What is an alternative method?