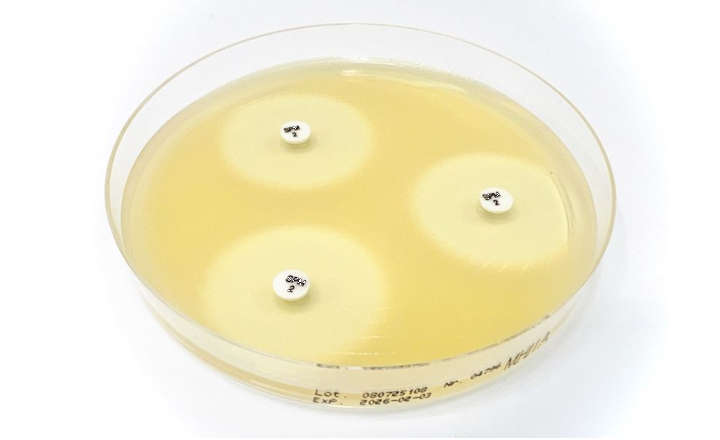
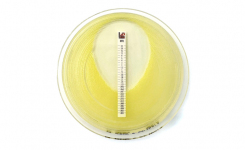
The Sulopenem SPM 2μg disc is intended to determine susceptibility of Enterobacterales to sulopenem, as recognized by the FDA Susceptibility Test Interpretive Criteria (STIC). Sulopenem at concentrations of 2μg should be interpreted at 16-18 hours of incubation.
The Sulopenem SPM 2μg disc demonstrated acceptable performance with the following Enterobacterales:
- Escherichia coli
- Klebsiella pneumoniae,
- Proteus mirabilis,
- Citrobacter freundii,
- Citrobacter koseri,
- Enterobacter cloacae complex,
- Klebsiella aerogenes,
- Klebsiella oxytoca,
- Proteus vulgaris,
- Providencia alcalifaciens,
- and Providencia stuartii.
Sulopenem at concentrations of 2μg should be interpreted at 16-18 hours of incubation.
Ref. 9262 250 discs (5 cartridges, 50/cartridge)
Please note : Any products described on this page are
for Research Use Only and not intended for clinical diagnostic procedures unless otherwise stated.