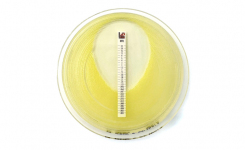
We're pleased to announce IVDR compliance for both our Cefepime-enmetazobactam MTS™ and disc products, reinforcing our commitment to regulatory excellence and laboratory quality.
Advanced Antimicrobial Susceptibility Testing
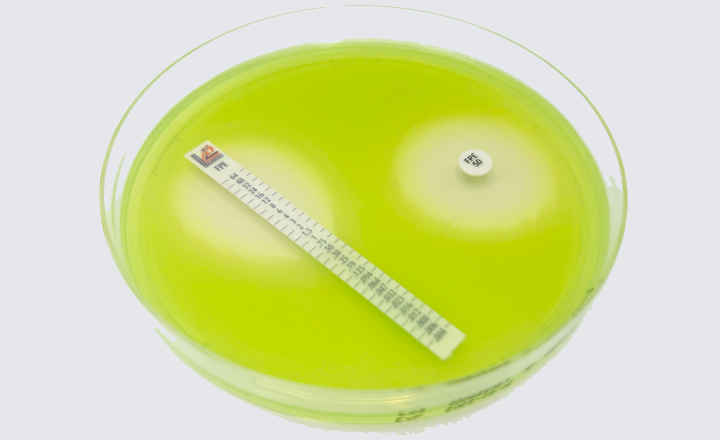
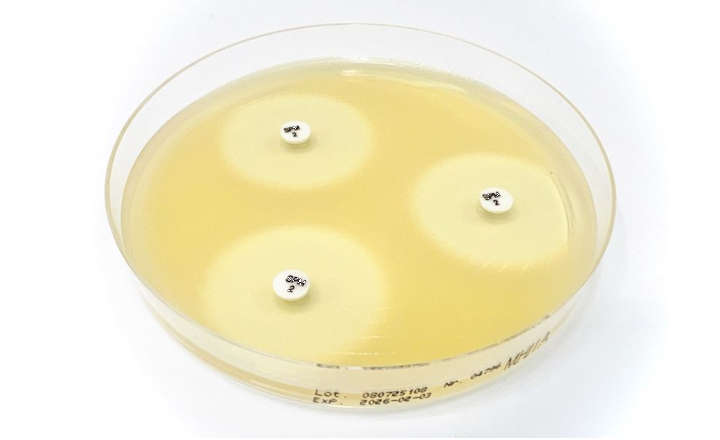
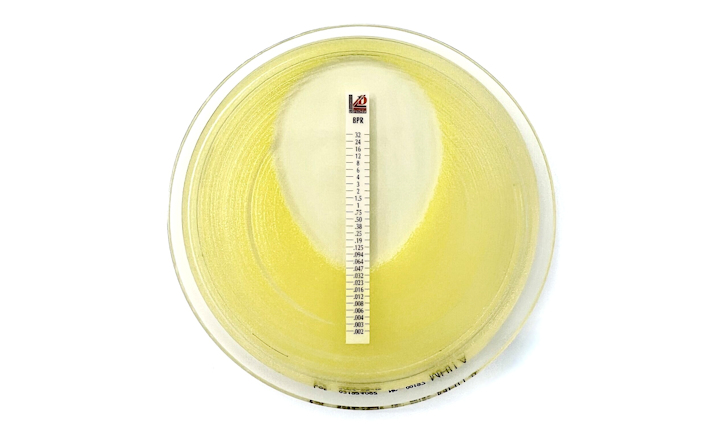
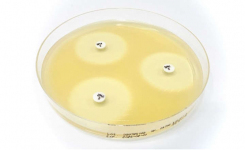
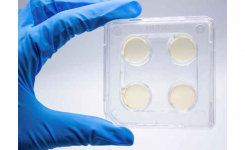
The Cefepime-enmetazobactam 0.004/8-64/8 MTS™ (MIC Test Strip) and (30+20) 50 μg disc provide reliable in-vitro quantitative and semi-quantitative methods for antimicrobial susceptibility testing of clinical isolates on agar media using overnight incubation.
Available Product Options:
MTS™ (MIC Test Strip): - Download product guide here.
- Ref. 920981: 10/pack (individually packed)
- Ref. 92098: 30/pack (individually packed)
- Ref. 920980: 100/pack (canister pack)
Disc: - Download product guide here
- Ref. 9270: 5 cartridges (50 discs/cartridge)
This compliance milestone ensures laboratories can confidently integrate these testing solutions into their quality control programs while meeting current regulatory standards. Cefepime-enmetazobactam MTS™ (MIC Test Strip) and disc are available in the United States as Research Use Only devices (RUO).
Learn More About Our IVDR-Compliant Solutions.