Bio-Rad Receives AOAC International Validation for EZ-Check Listeria spp. and EZ-Check Listeria monocytogenes Testing Kits
Bio-Rad Laboratories today announced it has received validation from AOAC International for its EZ-Check Listeria spp. and EZ-Check Listeria monocytogenes Kits for the detection of Listeria spp. and Listeria monocytogenes in food products and environmental samples.
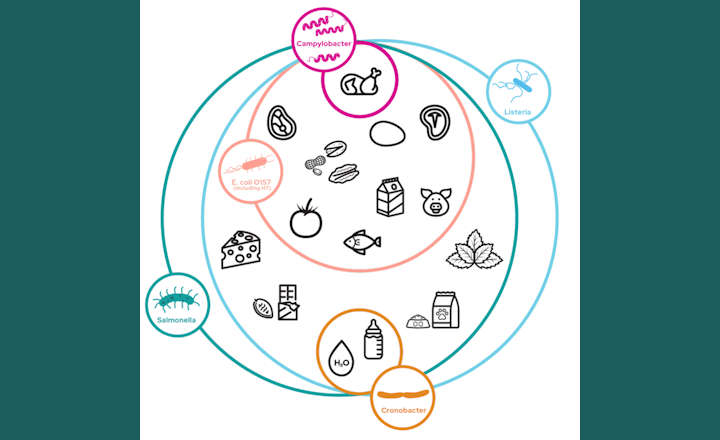
Listeria are gram-positive bacteria responsible for causing listeriosis. Primarily found in soil, water, and animal products, accurate detection of contamination in both food and environmental samples is critical in ensuring the production of safe consumables. Even with strict safety controls, contamination can still occur, posing health risks, costly recalls, and potential damage to brand reputation.
Bio-Rad’s ready-to-use, next-generation EZ-Check Listeria spp. and EZ-Check Listeria monocytogenes Kits deliver streamlined workflows with rapid time to results. Featuring multiplex amplification of the target pathogen and a critical internal control to monitor for false negatives, the proprietary, patented duplex detection system enhances accuracy and simplifies reaction set-up.
“Listeriosis is a serious infection that can be life threatening for pregnant women, children, and individuals with weakened immune systems, meaning accurate detection is of the utmost importance,” said Dr. Jesse Miller, Associate Director of Marketing, Food Science Division, Bio-Rad Laboratories. “The EZ-Check real-time PCR product range provides a premium and simple workflow, enabling rapid, reliable detection of key organisms. AOAC validation of the EZ-Check Listeria spp. and EZ-Check Listeria monocytogenes Kits further demonstrates our established position as leaders in food safety testing, delivering effective solutions for pathogen detection in food and environmental samples”.
For more information on Bio-Rad’s EZ-Check Kits
Bio-Rad will be showcasing the EZ-Check Listeria kits in an upcoming webinar series.
For more information and to register.