- Supporting the fight against AMR, Streck ARM-D® Kit OXA now provides effective detection of new variants
- The kits provide a cost-effective option for public health laboratories when used as a pre-WGS screening
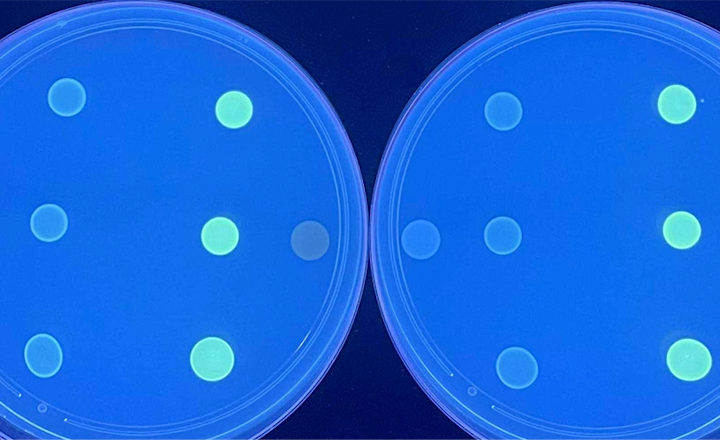
Streck has announced the release of an updated version of the Streck ARM-D® Kit, OXA that detects an additional 36 allelic variants in 3 new antimicrobial resistance (AR) gene families. This expanded coverage is in response to customer feedback, particularly the member laboratories of the Antimicrobial Resistance Laboratory Network (ARLN) and the Centers for Disease Control and Prevention (CDC), who have identified gene families and allelic variants of increasing diversity and relevance and require a surveillance method that can effectively detect them. Of particular interest to these labs is effective detection of the variants of OXA-235, OXA-236, and OXA-237 in the OXA-134 family, as well as OXA-327-like and OXA-198-like beta-lactamase enzymes.
“The expansion of the Streck ARM-D Kit, OXA, demonstrates our dedication to ensuring our products meet the needs of our customers in public health laboratories and emphasizes our commitment to make a global impact through solutions that improve infectious disease testing strategies,” said Chris Connelly, director of Business Segment - Molecular. “We’re proud to be a trusted resource for laboratories working hard in the fight against antimicrobial resistance.”
Streck’s ARM-D kits allow for the cost-effective and comprehensive detection of more than 2,000 allelic variants across 25 AR gene families. Results from the kits demonstrate 100% concordance with whole genome sequencing (WGS) data from the same samples, for the targeted AR variants. The ARM-D kits are often used as a pre-WGS screening method to reduce the cost of AR surveillance in public health laboratories in the United States and other parts of the world.