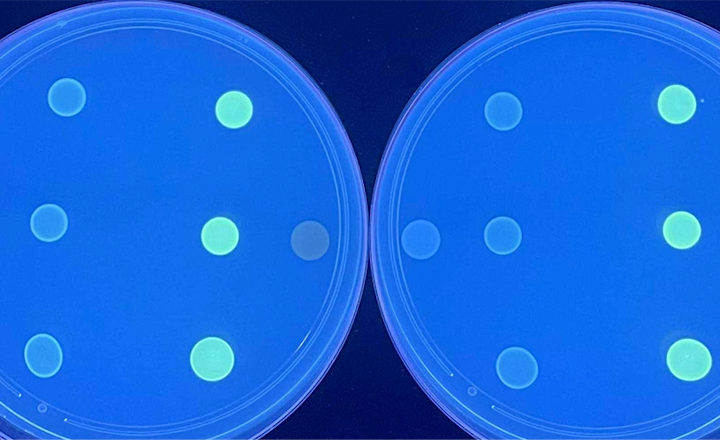
Molsid has announced the successful completion of the clinical performance study for its first diagnostic kit, ResiScreen® Staph. This milestone marks a major step toward the commercial launch of the company’s innovative solution for the reliable detection of β-lactam resistance in Staphylococcus strains.
The study was conducted in collaboration with the French National Reference Centre for Staphylococci (Hospices Civils de Lyon, France) under the supervision of Prof. Frédéric Laurent and Dr. Céline Dupieux-Chabert. It evaluated the performance of ResiScreen® Staph on 300 clinical isolates of Staphylococcus aureus and Staphylococcus non-aureus, confirming the kit’s robustness, reliability, and clinical relevance.
Accurate detection of staphylococcal beta-lactamase remains a diagnostic challenge as phenotypic methods, such as the benzylpenicillin disc edge test and nitrocefin-based assays, remain observer-dependent and can lack sensitivity. Powered by Molsid’s patented SmartID® fluorescent biotracer technology, ResiScreen® Staph overcomes the limitations of current methods for β-lactamase detection. It provides an easy-to-interpret binary readout with a broad-spectrum applicability to accurately detect blaZ-encoded penicillinase activity in staphylococci. The method is cost-effective, requiring minimal equipment and perfectly adapted to routine laboratory workflows. This capability represents a significant advance in the field of medical diagnostics, addressing a critical need for fast, reliable, and easy-to-interpret antimicrobial resistance detection in microbiology laboratories.
“This achievement represents a key milestone for Molsid, paving the way for the commercialization of ResiScreen® Staph,” said Stéphane Gavoille, CEO of Molsid. “We are proud to bring to market an innovative and practical diagnostic tool that will directly support clinicians and microbiologists in the global effort to contain antimicrobial resistance.”
With the successful validation of ResiScreen® Staph, Molsid reaffirms its mission to deliver next-generation diagnostic technologies that combine scientific excellence, clinical value, and operational simplicity. Eurobio Scientific will commercialize the product across key European territories.