Campylobacter in Foo...
Why Aren't We Ta...
18th June 2019 Content supplied by: Solus Scientific Solutions Ltd.
Solus One Salmonella: Simplify Your Spice Testing
Testing spices and herbs is notoriously difficult due to the antimicrobial properties intrinsic to many of these products. The Bacteriological Analytical Manual (BAM) Chapter 5 protocols for Salmonella testing require spices such as oregano, cinnamon, cloves and allspice to be diluted to beyond their toxic threshold, dilutions requiring enormous quantities of liquid media. This extreme diluting reduces the potency of the spice to a level that allows for the recovery and growth of any Salmonella that is present. The large media volumes involved, however, can potentially pose handling challenges for labs testing these matrices.
Solus Scientific are currently leading the way with alternative methods for testing Salmonella in herbs, spices and flavourings. Many alternative pathogen tests require modified media and differing incubation protocols in order to recover and detect the target organism in certain matrices. This could lead to confusion and mistakes occurring in testing labs when multiple different protocols are required for testing a variety of matrices.
Variation on a Theme
At Solus Scientific we have developed a simpler alternative. Solus One Salmonella immunoassay requires only 4 minor variations on a single protocol for all matrices, including herbs, spices and flavourings. All variations use a proprietary modified BPW + Solus selective supplement which enhances the recovery and growth of Salmonella.
The second variation is for larger sample sizes of herb and spice blends. The 375g sample is prepared at a 1 in 10 dilution. Although the volume of media required is greater than the standard protocol, the ratio of sample to media remains the same making the size of the sample the only deviation from the standard directions.
The third variation is specifically for oregano and cinnamon, both of which require 1:100 dilution for BAM protocols. With Solus One Salmonella immunoassay a 1 in 50 dilution is required for 25g samples, halving the amount of media needed for testing these products.
The fourth variation of Solus One Salmonella protocol is for cloves, the dilution of which for BAM is 1:1000. Again, Solus One Salmonella immunoassay dramatically decreases the volume of media required by needing only one tenth of the amount. Our media is selective, specific and sensitive enough to only require a 1 in 100 dilution for this potent ingredient.
AOAC Extension to Scope
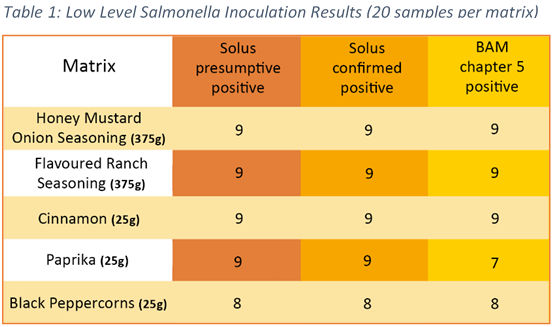
The development of this set of methods for herbs, spices and flavourings forms part of our application to AOAC for extension to scope for these matrices. Encouragingly, our results to date show a statistically insignificant difference between Solus One Salmonella presumptive and confirmed results and between Solus One Salmonella method and reference method at 5% level for all matrices evaluated. Table 1 shows a summary of results from the low level (0.2 – 2 CFU/ml) inoculation group of test samples.
Conclusion
Solus Scientific developed a proprietary modified BPW and selective supplement to enhance Salmonella detection in difficult to test matrices such as spices, herbs and flavourings. We established a set of 4 variations on a core Salmonella testing protocol using this Solus media and the Solus One Salmonella immunoassay.
By keeping our testing protocol suite simple, Solus Scientific hope to help analytical labs keep costs to a minimum through reducing the volume of media required for testing. The use of only 4 variations should also help to prevent possible human errors of incorrect sample preparation for Salmonella testing.
Solus Scientific are excited for the imminent extension to scope of Solus One Salmonella assay AOAC certification and we expect this to confirm us as the market leader in this testing arena.
Tags:
Date Published: 18th June 2019
Source article link: View
Campylobacter in Foods - a
Why Aren't We Talking