Liofilchem has received clearance from the FDA to market in the United States the MTS™ (MIC Test Strip) Plazomicin, a quantitative assay for determining the Minimum Inhibitory Concentration (MIC) of Plazomicin against Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis.
More technical details on the Plazomicin MIC Test Strip IFU
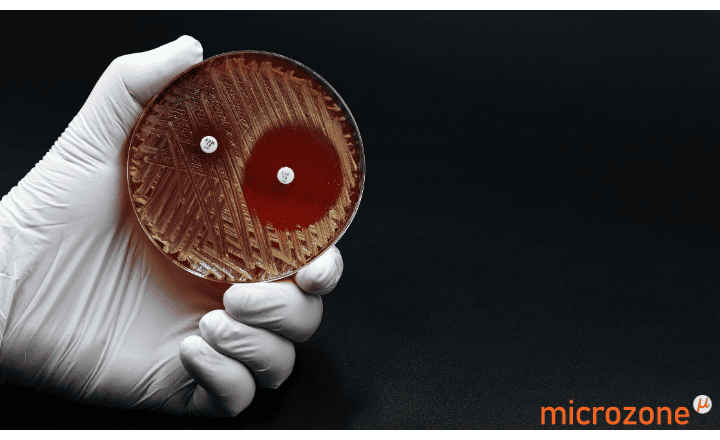
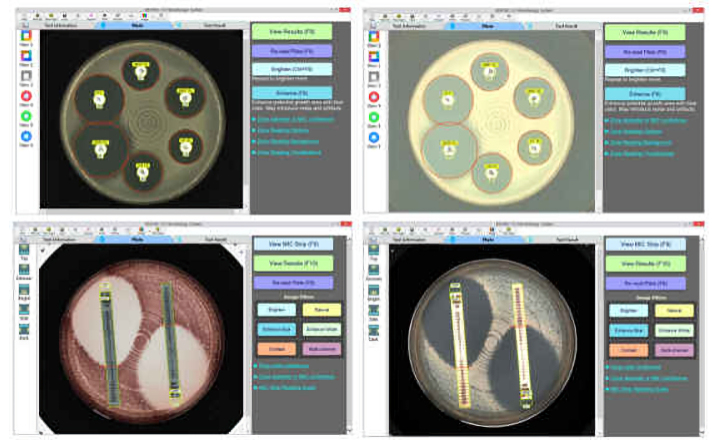
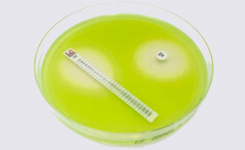
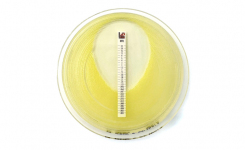
MTS™ (MIC Test Strips) are porous strips with special features (International Patent) that are impregnated with a predefined concentration gradient of antibiotic, across 15 two-fold dilutions of a conventional MIC method. On one side of the strip there is a printed MIC scale in μg/mL and a code that identifies the antimicrobial agent.
MTS™ Plazomicin 0.016-256 μg/mL
- 10 strip pack: ref. 920701
- 30 strip pack: ref. 92070
- 100 strip pack: ref. 920700
Plazomicin is the most recent FDA approved item in the MTS™ (MIC Test Strip) product range, following approvals of
- Vancomycin,
- Dalbavancin,
- Ceftolozane-tazobactam,
- Meropenem,
- Ceftazidime,
- Telavancin,
- Tedizolid,
- Delafloxacin,
- Clindamycin,
- Erythromycin,
- Linezolid and
- Meropenem-vaborbactam
- Azithromycin
- Ceftazidime-avibactam strips.
In the meantime, the rest of the MTS™ range is available in the United States as research use only devices, while the entire MTS™ product catalog is CE marked and fully available as IVD for clinical diagnostics purposes in Europe and approved in Canada by Health Canada. MTS™ is also registered at the competent Authority in many countries outside Europe as a clinical diagnostic device.
The current MTS™ range comprises antibiotics, antifungals, antimycobacterials, and combined strips for detection of resistance mechanisms (ESBL, MBL, KPC, AmpC and GRD) for over 150 items, each available in three pack formats (10, 30 and 100), for the widest gradient strip range in the international market.