Liofilchem has received clearance from the FDA to market in the United States the Dalbavancin MIC Test Strip, a quantitative assay for determining the Minimum Inhibitory Concentration (MIC) of dalbavancin against Staphylococcus aureus (including methicillin resistant strains) and Enterococcus faecalis (vancomycin susceptible strains).
More technical details on the Dalbavancin MIC Test Strip IFU: www.liofilchem.net
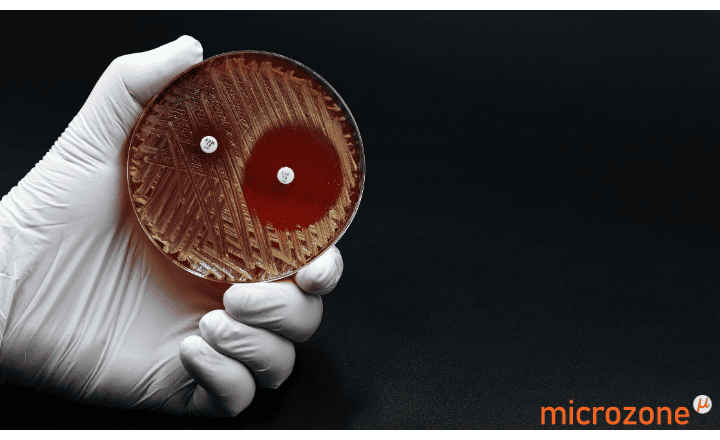
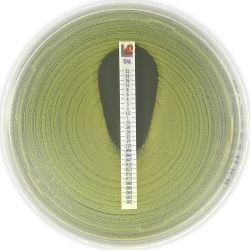
MIC Test Strips are porous strips with special features (Italian Patent no. 1395483 and International Patent pending) that are impregnated with a predefined concentration gradient of antibiotic, across 15 two-fold dilutions of a conventional MIC method. On one side of the strip there is a printed MIC scale in μg/mL and a code that identifies the antimicrobial agent.
Dalbavancin MIC Test Strip 0.002-32 μg/mL
- 10 strip pack: ref. 921371
- 30 strip pack: ref. 92137
- 100 strip pack: ref. 921370
Dalbavancin is the second of a long series of MIC Test Strip items soon to be approved by the FDA, following recent approval of the Vancomycin strip. In the meantime, the rest of the MIC Test Strip range is available in the United States as research use only devices, while the entire MIC Test Strip product catalog is CE marked and fully available as IVD for clinical diagnostics purposes in Europe and approved in Canada by Health Canada. MIC Test Strip is also registered at the competent Authority in many countries outside Europe as a clinical diagnostic.
The current MIC Test Strip range comprises antibiotics, antifungals, antimycobacterials, and combined strips for detection of resistance mechanisms (ESBL, MBL, KPC, AmpC and GRD) for over 150 items, each available in three pack formats (10, 30 and 100), for the widest gradient strip range in the international market.