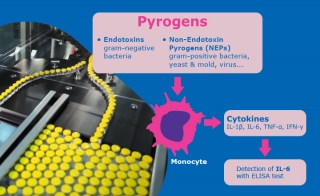
How much confidence have you in your current pyrogen and bacterial endotoxin test methods? What's your thoughts on moving away from rabbit pyrogen tests or LAL to non-animal-based testing, such as the Monocyte Activation Test (MAT) and recombinant Factor C (rFC)?
Whether you're happy with the status quo or considering a changing of the guard, you'll find this comprehensive special focus on endotoxin & pyrogen (non-endotoxin) testing of benefit. Get in touch with suppliers today and ask for that free consultation or brochure, to be sure you are using the most suitable kits for the safe release of your company's parenterals and medical devices as implants.
Evaluating Pyrogen Contamination Risk and the Need for the MAT in Pharmaceutical Processing
Authored by Tim Sandle, BPL, and Laure Robert, Merck, this report considers risk contamination coming from non-endotoxin pyrogens (NEP). Risk assessment of NEP is discussed and advantages of the Monocyte Activation Test (MAT), as opposed to the Rabbit Pyrogen Test or the BET, are explored.
Find Out More
Explore Bacterial Endotoxin Testing from Charles River
Charles River’s Endosafe® portfolio of FDA-licensed products for bacterial endotoxin testing is designed to increase data integrity compliance, reduce retest rates, and streamline manufacturing processes, allowing prompt, confident decisions.
Find Out More
Why MAT Will Save Rabbits and Product Recalls - A rapidmicrobiology Podcast
In this episode, Laure Robert (Global Product Manager with Merck KGaA Darmstadt, Germany) emphasizes the importance of pyrogen testing with MAT as a final release test, gives reasons for geographical differences in MAT standardization, and describes how to implement MAT in the lab, step-by-step.
Find Out More
The Next Chapter . . . Lonza’s Endotoxin and Pyrogen Testing Solutions
Modern QC testing includes traditional and sustainable methods that can be automated. Lonza’s expert support and complete testing portfolio provide your laboratory versatile solutions to meet your current and future pyrogen and endotoxin testing needs.
Find Out More
Breakthrough Endotoxin and NEP Test for Life Changing Products
Successful Monocyte Activation Testing (MAT) of all parenteral pharmaceuticals, biologicals and medical devices; market leading assay sensitivity (LoD 0.004 EU/ml); 2,500 vials per PBMC batch; successful LER effect curbing; worldwide GMP certified MAT services.
Find Out More
Proficiency Testing: A Useful Quality Control Tool for Pharmaceutical Laboratories
BIPEA offers three PT programs - Purified water for pharma applications: Endotoxins and TOC in purified water, Residual solvents in excipient: Ethylene oxide and Dioxane in Polyethylene glycol, Microbiology of pharmaceutical process water: total viable aerobic count in water.
Find Out More
FUJIFILM Wako Chemicals U.S.A., Endotoxin-Specific LAL Reagents
With over a century of combined research experience, FUJIFILM and Wako Chemicals U.S.A. continue the development of products that can improve human health and protect the environment. See the complete line of Wako LAL products at wakopyrostar.com
Find Out More
10 Reasons to Choose rFC Bacterial Endotoxin Test - a bioMérieux White Paper
This whitepaper compares the performance of LAL reagents with rFC, and shows the evidence supporting the 10 reasons to choose recombinant horseshoe crab Factor C (rFC) for your bacterial endotoxin testing.
Find Out More