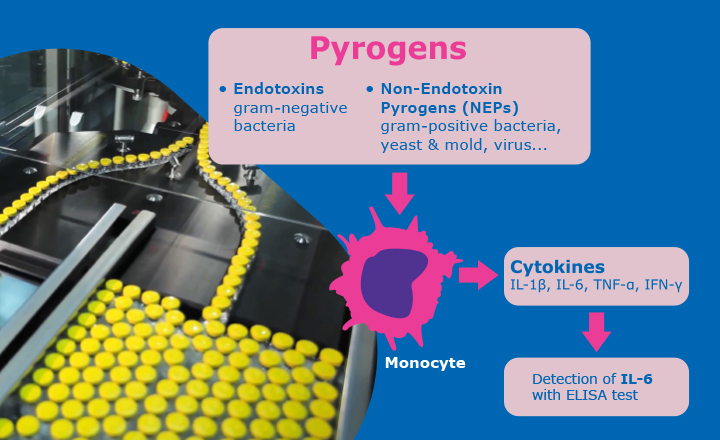
Pyrogens are fever-producing substances. Microorganisms such as Gram-negative or Gram-positive bacteria, viruses, and fungi contain metabolic components that can potentially activate the innate immune system. These components can occur independently of viable microorganisms and are a safety concern in parenterally administered drugs since they can cause severe reactions such as fever, rash, headache, myalgia, nausea, vomiting, organ failure, and shock in the recipient. For this reason, the measurement of pyrogens is an important safety measure for parenterally applied drugs.
Pyrogens are commonly divided into two classes: exogenous pyrogens, such as endotoxin from Gram-negative bacteria that induce fever when applied intravenously; and endogenous pyrogens that are induced inside the body as a reaction to the contact with exogenous pyrogens and cause an elevation in body temperature (endogenous pyrogens have potent pyrogenic and inflammatory activities and include interleukin 1-a (IL-1a), interleukin-1b (IL-1b), tumour necrosis factor a (TNF-a) and interleukin-6 (IL-6)).
In this report, non-endotoxin pyrogens from exogenous source and their associated risks are the subject.
Beginning with Gram-positive microorganisms, one pyrogenic component are teichoic acids and lipoteichoic acids (LTA), as might be found, for example, within Staphylococcus aureus or Streptococcus faecalis. Studies have found the potency of LTAs to be significantly lower than that of the LPS or even inactive. This infers that a high Gram-positive bioburden would need to be present in order to trigger a cytokine response. To assess this bioburden data requires review, including speciation. Another cell-related material that can be pyrogenic is peptidoglycan. Relatively high numbers of Gram-positive bacteria are required to trigger a pyrogenic response (at around 108 cells). As well as conferring a level of heat resistance to the cell, peptidoglycan, as a cellular component can remain after sterilization by heat. With sterilization by filtration, should cells be destroyed, then as a cellular substructure, peptidoglycan polymers are small enough to pass through a sterilizing grade filter.
Other pyrogenic substances comprise bacterial exotoxins, which can trigger toxic shock-like syndrome, characterized by hypotension or shock, fever, multiorgan system involvement. Among the best-characterized exotoxin, superantigens are the family of pyrogenic exotoxins produced by Staphylococcus aureus and Streptococcus pyogenes. Exotoxins have a degree of heat resistance. Another microbial source are enterotoxins, e.g. from Staphylococcus aureus. Enterotoxins are single-chain globular proteins of varying molecular weights. It is not clear what level of enterotoxin is needed to elicit a pyrogenic response. Monitoring for high levels of staphylococcal bioburden (especially Staphylococcus aureus) needs to be a factor for risk control.
Fungi can produce pyrogenic reactions (through the release of mannans, glucans, and mannoproteins). While this may depend on the type of fungi, numbers of fungal cells approaching the 1000s are required to trigger a response. Assessing environmental monitoring data is important to assess the levels of fungi. Pyrogenic responses can also occur in relation to viruses. Many types of virus can elicit a pyrogenic response in people (for example, rhinovirus infections). The degree to which this presents a concern depends on whether viral removal or inactivation steps are present in a pharmaceutical process and which can abolish haemagglutinin activity.
Pyrogens may be present in the water used as the solvent or in the processing; or in ingredient raw materials. These sources need to be evaluated along with any mitigation methods, for example, with water sourcing Water-for-Injections and using ultrafiltration can remove some pyrogens through size exclusion. Specific types of raw materials should be examined to determine the potential for the presence of pyrogens.
Currently, and as approved by official compendia, there are three testing possibilities available to test for pyrogens: the Rabbit Pyrogen Test, the Bacterial Endotoxin Test (primarily the Limulus Amoebocyte Lysate (LAL) test), and test systems using human whole blood or human monocytes. The latter is called the Monocyte Activation Test (MAT). The European Medicines Agency (EMA) has encouraged the replacement of the rabbit pyrogen test by alternative tests such as MAT or LAL. This forms part of the 3Rs principle – the replacement, reduction, and refinement of animal testing. In relation to animal tests, EMA states:
“With a view to their replacement by in vitro methods wherever possible, in accordance with the European Convention on the Use of Animals for Experimental and Other Scientific Purposes and with EU Directive 86/609/EC. The revised monograph introduces a provision for use of an in vitro method as a preferred alternative to the pyrogen test in rabbits”.
Whether LAL itself is sufficient depends upon the risk posed by other pyrogenic substances. Regulators require a risk assessment to be conducted where the pharmaceutical process is assessed, beginning with incoming raw materials and ending with the final formulated bulk.
To determine the risk of non-endotoxin pyrogens entering the final drug product, , a formal assessment is required, including:- Likelihood of pyrogenic substances of microbial origin and non-microbial origin.
- Assessment of controls relating to material and equipment providers.
- Assessment of the presence of pyrogenic substances in the manufacturing process.
- Review of pyrogen removal steps.
- Type and history of the finished product.
The outcome of this assessment will determine whether the MAT is required.
Unlike the LAL test, the MAT is a powerful in vitro test for the detection of both endotoxin and non-endotoxin pyrogens (NEPs) acting via the toll-like receptors (TLRs) pathway. Therefore, MAT constitutes a safe test for parenteral drug release when the risk assessment has highlighted potential sources of NEPs entering the production process.
In addition to reducing the need for animal-based tests, MAT provides several advantages over the rabbit pyrogen test. First, it relies on the human immune reaction instead of an animal model, reflecting the actual pyrogenicity of contaminants in the human blood.
It is also a more sensitive and more robust test, available in ready-to-use formats for an easy implementation in the Quality Control laboratory.
The various available kits use different sources of monocytes. The solutions are based either on cryopreserved whole blood, peripheral blood mononuclear cells (PBMC), or monocytes from the Mono-Mac-6 cell line (MM6).
The MM6 cell line, which has been validated for use in the MAT, has sometimes been questioned over its ability to detect non-endotoxin pyrogens (NEPs). To demonstrate its performance, tests have successfully been performed using a wide panel of TLRs ligands.
The PyroMAT® System is a ready-to-use MAT, using the MM6 cell line as a source of monocytes for pyrogen detection. It is a valuable test method to replace the rabbit pyrogen test or the LAL test for the batch release of parenteral drugs.
Learn more about the in vitro PyroMAT® system on SigmaAldrich.com/pyromat
If you have any questions, please contact us.
About the authors:

Dr. Tim Sandle is Head of Microbiology, Quality Risk Management, and Sterility Assurance at BPL and a visiting tutor at the University of Manchester and University College London. Dr. Sandle has written 30 books and over seven-hundred publications relating to microbiology, pharmaceutical and healthcare sciences. Dr Sandle’s microbiology website is: www.pharmamicroresources.com

Laure Robert is a Product Manager working within the BioMonitoring organization of Merck Life Science. She took this role in 2017 and has been in charge of pyrogen testing solutions since then. During this experience, she had the opportunity to launch a new monocyte activation test solution on the market, namely PyroMAT® System.
Before joining Merck, she had experience as a product manager within the pharmaceutical industry for two years and then held a position of consultant within the healthcare sector for two more years. She holds a Pharm. D degree obtained at the University of Lille, France, and a Master’s degree in Business Management (EDHEC Business School).