The pharmaceutical industry's COVID-19 pandemic response to develop a vaccine and supply drugs for patients has been a global collaborative effort. As giants of the industry merge with vaccine developers, the science community is witnessing a revolution in biologics manufacturing, where processing time is cut in half and operational efficiency as a whole, is maximized. And a significant factor in this encouraging response is the utilization of rapid microbial methods (RMMs). Whether it's for environmental monitoring, endotoxin or bioburden testing there's a role for faster, more efficient approaches.
In this rapidmicrobiology special focus, browse through a range of rapid methods that can help streamline the microbial testing workflow in vaccine development and production. We have an interview with Anne Connors, Senior Field Marketing Specialist at Merck, who discusses how the Milliflex® Family of rapid microbial methods allows faster testing of raw materials to aid final product release, and how customer feedback can drive innovation in providing a more user-friendly experience for the QC microbiologist. Discover the extensive validation support Charles River Laboratories are offering with their Celsis® rapid detection platform, that can deliver sterility testing in just 6 days.
Not sure what or how to test for endotoxin contamination? Allen L. Burgenson, Lonza Pharma&Biotech – Testing Solutions can guide you.
There's a downloadable study describing how Monocyte Activation Testing detects both endotoxin and non-endotoxin pyrogens in vaccine preparation. Plus, learn about FUJIFILM Wako's PYROSTAR™ technology, the only inherently endotoxin-specific products on the market and explore the differences between using LAL and ENDONEXT recombinant Factor C (rFC), with the downloadable paper here.
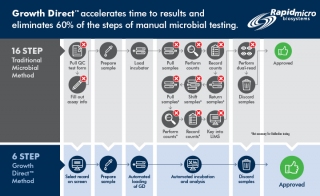
For environmental monitoring, BIOTRAK® can offer real-time viable particle counting for continuous manufacturing without stoppages. There's a useful infographic from Rapid Micro Biosystems clearly illustrating the differences in time to result and number of manual handling steps when using the Growth Direct™ system. Plus, Microgenetics are currently offering SmartControl environmental monitoring software free of charge for a 12-month period to facilities involved in the fight against COVID-19.
Sino Biologicals have a full range of bioreagents to compliment and aid in COVID-19 vaccine development including mammalian cell-made COVID-19 viral recombinant proteins.
Accelerating Pandemic Response Means Accelerating Release Testing
The world needs pharma and biotech organizations to respond to public health crises faster than ever. As production timelines shorten and approvals are fast-tracked, the industry must be able to release products as quickly and safely as possible.
Find Out More
Streamline Your Microbial Testing Workflow to Increase Vaccine Productivity
Anne Connors, Senior Field Marketing Specialist, Merck talks about how the Milliflex® Family of rapid microbial testing products, can reduce risk of cross-contamination, ensure better accuracy, provide a user-friendly experience for the microbiologist and aid in traceability and data integrity.
Find Out More
Let’s Step Forward to Meet the Next Challenge
This article describes how the endotoxin testing requirements for all drug products must be applied during development of vaccines. These tests help ensure the release of safe and effective therapeutics, including those targeting COVID-19 disease.
Find Out More
Study: MAT Detects Endotoxin & Non-Endotoxin Pyrogen in Vaccine Prep
The study shows that the PyroMAT® System, a monocyte activation test (MAT) that uses cryo-preserved Mono-Mac-6 (MM6) human monocytic cells as monocyte source, can detect both endotoxin and non-endotoxin pyrogen in a meningococcal vaccine preparation.
Find Out More
FUJIFILM Wako Chemicals U.S.A., Endotoxin-Specific LAL Reagents
With over a century of combined research experience, FUJIFILM and Wako Chemicals U.S.A. continue the development of products that can improve human health and protect the environment. See the complete line of Wako LAL products at wakopyrostar.com
Find Out More
Rapid Micro Biosystems Announces Global Expansion to Support Pharma Manufacturing
Learn how Rapid Micro Biosystems is expanding to meet the growing demand for their automated microbial detection platform Growth Direct™ See the system and discover how to eliminate 60% of the steps of manual testing methods, accelerate time to results and prevent human error in the QC lab.
Find Out More
Bioreagents Related to SARS-CoV-2 (COVID-19) Vaccine Development
To address this global public health crisis, unprecedented efforts are being made to study the culprit virus, SARS-CoV-2. Sino Biological is working to develop rapid diagnostic reagents, vaccines, and therapies against this virus.
Find Out More
Ecological, Efficient and Sustainable Endotoxin Testing
Vaccines can be complex matrixes and can cause difficulties when establishing and testing for Endotoxins. The Recombinant Factor C protein in ENDONEXT assays can be measured perfectly to contain only the needed Factor C protein for ENDOTOXIN detection.
Find Out More
Confidence Comes With a Higher Caliber of Data - BIOTRAK® Real-Time Viable Particle Counter
Implementation of the BioTrak Real-Time Viable Particle Counter in fill-finish spaces eliminates the need for process interruptions for environmental microbial monitoring. In many cases, payback time can be less than one year even when considering maximal costs of implementation.
Find Out More
Challenges of Environmental Monitoring: Scale-up with SmartControl
For facilities scaling-up to develop COVID-19 vaccines, environmental monitoring could be a challenge. Find out how software solutions such as SmartControl can help, and how you can get the software free of charge for 12-months if involved in the fight against the virus.
Find Out More
Bakers' Specialised Clean Air Enclosures Offer the Highest Standard of Air Containment
Baker continues to develop specialised walk in and reach in clean air enclosures ideal for vaccine developers, making innovative use of robotic technology for drug discovery, high-throughput screening, and high-volume sample management.
Find Out More