Rapid microbial methods (RMM’s) will play a critical role in the new normal. However, just being rapid isn’t enough. Your RMM needs to be the Right Microbial Method. Not all RMMs are created equal, and neither are suppliers. So how do you know you’re making the right decision for your products, your lab, and ultimately your company?
With the Celsis® rapid detection platform for rapid sterility, we’ve created an unprecedented set of solutions, features, and options in a package that’s designed for simplicity, not complexity. A factor that’s become more critical than ever.
In order to help meet the demand of a global population in need of lifesaving therapies, it’s more important than ever that vendors become more than just instrument suppliers. They need to become partners: an organization to work alongside with you every step of the way.
Celsis Complete and Celsis Advantage Support Packages for Rapid Sterility
Two support packages are now available to demonstrate validation parameters described by USP , Ph. Eur 5.1.6, and PDA TR 33, providing a complete solution that reduces the burden of validation for Celsis® rapid detection, allowing your staff and resources to focus on production:
- Celsis® Complete Validation & Reports
- Validation Documentation including Sample Effects Testing Protocol, Equivalency Validation Report, Ruggedness & Robustness Technical Reports.
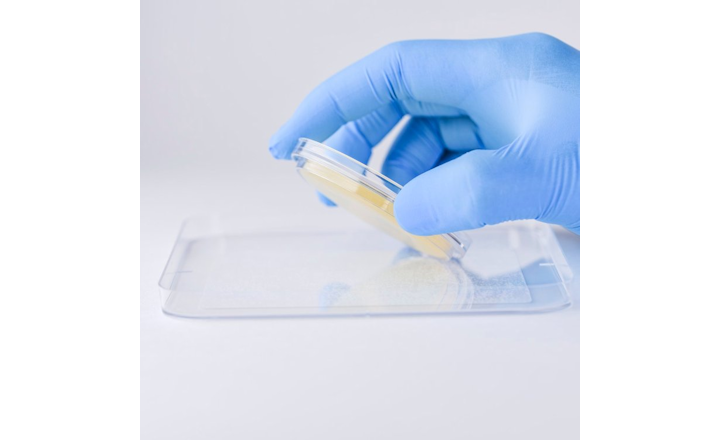
- Testing in presence of your product, performed by Charles River, for Method Suitability, Equivalency, Limit of Detection and Specificity.
- Celsis® Advantage Reports & Protocols
- Validation Documentation including Sample Effects Testing Protocol, Equivalency Validation Report, Ruggedness & Robustness Technical Reports.
- Test methods and protocols, provided by Charles River, for Method Suitability, Equivalency, Limit of Detection and Specificity.
We’ve harnessed the power of our global support network, our entire portfolio and our industry-proven expertise to provide custom tailored validation support options depending on your strategy, your needs, and your products. Simple answers to a complex question.
For more information, please visit criver.com/rapidsterility or contact Celsis-Support@crl.com to start the conversation.