Extended Matrix Vali...
CloudLIMS’ New...
23rd April 2019 Content supplied by: Solus Scientific Solutions Ltd.
Out With the Old, in With the… Old?!
Article by: Celia Holdsworth, Product Specialist for Solus Scientific
Is ELISA an outdated technology?
Technology is constantly changing and evolving. What was once top of the range is soon relegated to the bottom of the bin and quickly forgotten. But does that mean that the original idea was bad? Should we completely turn our backs on it to look for the shiny new toy? At Solus, we don’t think so, because with just a few tweaks and upgrades, that “old” technology can become the next big thing.
Research and Development
The constant battle to be the one with the newest technology drives innovation and demands imagination and creativity. That’s where the Solus Research and Development team come in.
ELISA was first invented in 1971 and was originally used in a clinical setting as a rapid way of detecting hormones and viruses in patients. Although Solus assays use the same principle, they have been specially designed and developed for the food testing industry, making them much more suited to this particular niche.
Our R&D team has the perfect balance of knowledge, skill and enthusiasm. With expertise in antibody selection and purification, assay design and development, project management, and creativity, the Solus R&D team hold the key to our products’ success.
Not only do they carry out the assay development work, but they are also responsible for getting our assays accredited by AFNOR and AOAC, along with carrying out in-house validation work on the side.
So why did Solus make ELISA impressive again?
We listened to our customers. They told us what they wanted from a pathogen detection system and the Solus R&D team got to work.
We went back to basics and started building from the ground up. As a result, all of the key components of the ELISA have been upgraded. The growth media is better, the antibodies are better, the wash buffer is better, the conjugate and substrate is better, even the technique used for coating the plates with antibodies has been dramatically improved! We now have a system that has superior sensitivity without compromising on selectivity: the Solus One range.
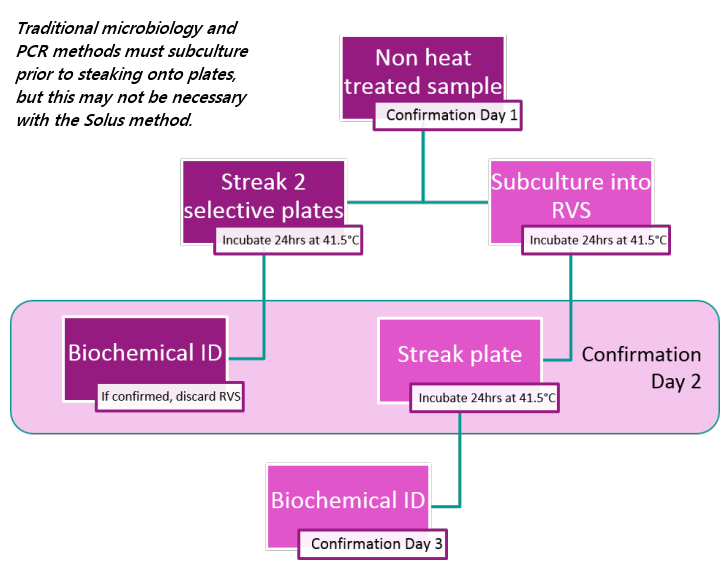
The Solus One range provides negative and presumptive positive results in a single day. Even taking into account confirmation steps for presumptive positive samples, the Solus One range offers results at least a day faster than traditional microbiology and PCR-based pathogen test confirmation techniques.
And the response has been staggering! There has been so much interest and enthusiasm for our new assays that we are already working on increasing the scope of accreditation for our Solus One range, as well as adding new assays to our collection to better fit the needs of our global clientele.
Following on from the recent development of the Solus One range, the Solus Scientific R&D team are bringing to the market new assays which will enhance our position in the field of food pathogen safety testing. This is an exciting time of growth with the R&D team as we continue to utilise our expertise.” comments Dr. Nevin Perera, R&D Manager.
With the power of automation, including full traceability from reception to result provided by the DS2’s on-board barcoding system, global food manufacturers and retailers are clamouring to be the first to provide faster results to their clients.
ELISA is dead. Long live ELISA!
We may base our assays on what some say is outdated technology. But we haven’t just accepted it for what it is. We’ve pushed it, we’ve tested the boundaries, and we’ve developed and improved it to become the powerful pathogen detection tool that the global food testing world is demanding.
If you want to be part of the new ELISA era then click this link for more information about the Solus One pathogen testing range.
ABOUT SOLUS™
Solus Scientific pathogen systems have been specifically developed with the constraints of a food testing environment in mind. Committed to food safety excellence Solus assays bring significant productivity benefits to the labs who employ them. Solus systems use proven, industry standard immunoassay technology that is easy to implement and use. Founded in 2009 the Solus vision was to develop pathogen testing immunoassays with rapid time to result. The first dual enrichment pathogen kits completed AFNOR certification in 2013. Solus Pathogen immunoassays are manufactured within our UK production facility to the highest standards, which are tightly controlled within our ISO 9001 accredited quality system.
Tags:
Date Published: 23rd April 2019
Source article link: View
Related news
Extended Matrix Validations for 3M&
CloudLIMS’ New Document Management