In the face of the escalating mpox outbreak, health authorities worldwide are on high alert, particularly with the emergence of the more contagious and aggressive clade Ib. The surge in cases has intensified the need for rapid and accessible diagnostic tools to curb the spread of the virus.
In response to this challenge, Fapon Biotech, a world-leading in-vitro diagnostics (IVD) total solution provider, has been in close communication with related organizations such as Africa Centres for Disease Control and Prevention (Africa CDC) and the Foundation for Innovative New Diagnostics (FIND) to discuss better diagnostic solutions. Fapon Biotech has not only upgraded its mpox antigen detection products but also meticulously validated their performance against positive samples with an authoritative institution. The upgraded product achieves a breakthrough detection limit of below 1 pg/mL for both Clade I and II recombinant antigens.
The validation experiment results have confirmed the product's performance in detecting diluted mpox virus culture samples at a minimum titer of 6.25 Pfu/mL (with a Ct value of 30 verified by a team of virologists from a leading national clinical research institution in China).
Clinical validation results of 22 clinical specimens from patients with mpox showed that the majority of samples were detectable, and judging from the chromatographic color intensities of the detected specimens, the detection Ct values were also around 30.
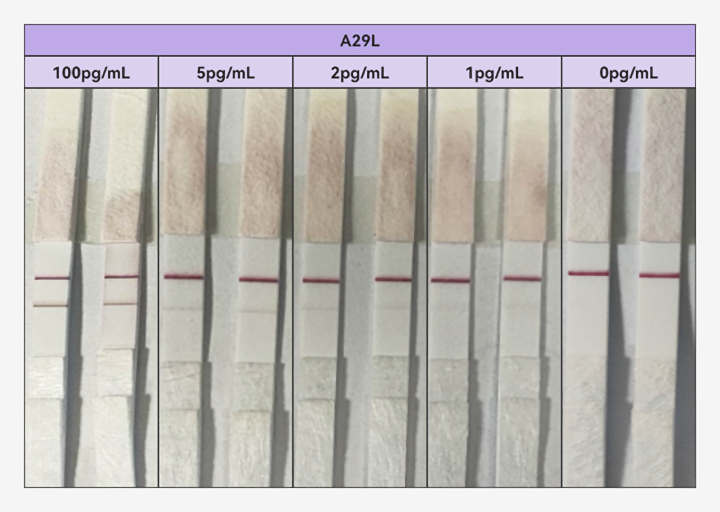
(Fapon's mpox antigen detection product for the detection of recombinant antigen A29L)
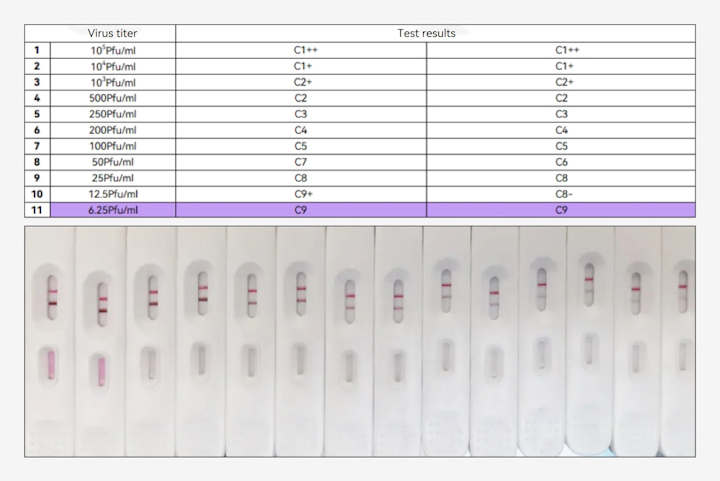
(Fapon's mpox antigen detection product for the detection of mpox virus culture fluid )

(Fapon's mpox antigen detection product for the detection of clinically positive samples of lesion surface swabs)
This rapid and convenient diagnostic tool is poised to make a significant impact on managing the outbreak in affected regions, particularly in regions where healthcare facilities may be limited. On August 29, the WHO called for global IVD manufacturers to submit applications for Emergency Use Listing (EUL) to expedite the rapid access to diagnostic tests for mpox. FIND has also issued a Request for Proposals (RFP) for mpox diagnostics, offering additional resources to support performance evaluation and accelerate product development.
Our Point-of-Care Testing (POCT) antigen detection products offer the speed and convenience essential for effective disease surveillance and containment. For more information on how Fapon Biotech can support your efforts or to initiate a partnership, please contact us using the green "Request Information" button below. Together, let us harness the power of innovative diagnostics to protect and preserve the health of communities across Africa and beyond.