Foodborne pathogens can contaminate food in several ways, for example, through insufficient hand hygiene of personnel, coming into contact with other contaminated surfaces during production, or through improper storage or handling during transportation from the production facility to the consumer.
In recent years, there have been high-profile outbreaks of foodborne illnesses caused by surface contamination. For example, in 2019, a Listeriosis outbreak was linked to contaminated deli meats and cheese, which were found to be contaminated from surfaces and slicing equipment [11]. Similarly, a Salmonella outbreak linked to contaminated papayas in 2017 was traced back to the surfaces of papaya processing equipment in the packing facility[4]. In 2018, an E. coli outbreak was also linked to contaminated romaine lettuce, which was found to be contaminated from surfaces in the processing and packing facilities[3]. These examples highlight the importance of proper sanitation and hygiene practices throughout the food production and distribution process to prevent the spread of foodborne illnesses.
Early detection of foodborne pathogens in the processing and handling environment can help prevent outbreaks of foodborne illness and minimize the risk of product recalls[5]. By monitoring surfaces, equipment, air, and water, potential contaminants and areas of risk can be identified, and corrective actions (e.g., modifying cleaning and sanitation strategies and reducing transmission pathways) can be taken to prevent contamination of the food[2].
Choosing the right hygiene testing technology is, sometimes, not a trivial decision. Conventional analytical techniques based on the detection of biochemical and microbiological constituents are reliable and still in operation.- Cultural methods: These methods involve the use of selective and differential media to culture microorganisms. It is still considered the Gold Standard due to its reliability, basic visualization, and ease of use[1].
- Real-Time Polymerase Chain Reaction (Real-Time PCR): The highlight of this technique is its sensitivity and the ability to screen a considerable number of pathogens simultaneously[12], and this is the reason why PCR-based assays for the detection of viable pathogens in foods have increased significantly in the last 15 years[13].
- Enzyme-Linked Immunosorbent Assay (ELISA): is considered a valid rapid, and easy alternative to conventional methods able to detect this pathogen in food[6].
- Emerging new pathogens detection techniques (next-generation sequencing and biosensors) are gaining importance due to their rapidness, sensitivity, specificity, and high throughput[10] [7].
Neither the choice of the technology nor the sampling areas or frequency of testing are strongly defined, as it depends on the characteristics of the processing plant[8]. There are several food safety regulations and guidelines that outline the requirements for risk-based environmental monitoring of foodborne pathogens: FDA Food Safety Modernization Act (FSMA), ISO standard, and Good Manufacturing Practices (GMPs). In addition, there may be specific requirements at the national, state, or local level, depending on the location of the facility and the products it produces. It is important for food processing and handling facilities to be familiar with the applicable regulations and guidelines and to implement appropriate environmental monitoring programs to ensure food safety.
The probability of contamination of the sample or product, its historical contamination data, potential consequences on human health, sampling area location, external factors such as the incoming goods, etc., are many of the considerations that need to be taken into account to design an optimal risk-based environmental monitoring[9].
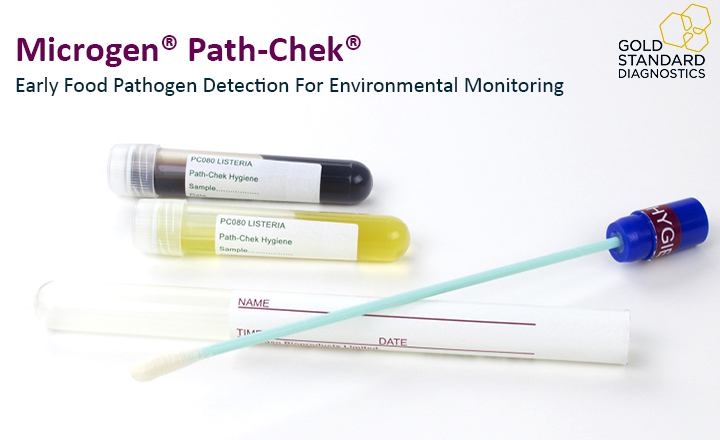
For that reason, Gold Standard Diagnostics has expanded the product range of pathogen detection with the Microgen® Path-Chek® product line, including Path-Chek® Hygiene Swab System (for Salmonella, Listeria, and Coliforms), Path-Chek® Hygiene Protein and Path-Chek® Heat Bath. It complements the BACGene molecular diagnostic offering (sensitive and certified) with a simple and cost-effective approach for environmental monitoring.
Key benefits of Path-Chek® products:
- Save space and costs: no further equipment other than an incubator (Path-Chek® Heat Bath) is needed.
- Save time: easy handling - results for Path-Chek® Hygiene Protein in only 5 seconds.
- Pre-moistened swabs give improved recovery on both wet and dry surfaces and ensure the integrity of the sample.
- Easy interpretation: the presence of specific target bacteria causes the colour change of the media.
Please click here for more information about our Microgen® Path-Chek® product range or contact Gold Standard Diagnostics for more details using the green "Request Information" button below.
Bibliography
[1] Abayasekara L.M., P. J. (2017). Detection of bacterial pathogens from clinical specimens using conventional microbial culture and 16S metagenomics: A comparative study. BMC Infect. Dis., 17:631. doi: 10.1186/s12879-017-2727-8.
[2] Barnett-Neefs C, S. G. (2022). Using agent-based modeling to compare corrective actions for Listeria contamination in produce packinghouses. PLoS ONE 17, https://doi.org/10.1371/journal.pone.0265251.
[3] Bottichio L, K. A. (2020). Shiga Toxin-Producing Escherichia coli Infections Associated With Romaine Lettuce-United States, 2018. Clin Infect Dis., 71(8):e323-e330; doi: 10.1093/cid/ciz1182. PMID: 31814028.
[4] Brooke M. Whitney, M. M. (2021). A Series of Papaya-Associated Salmonella Illness Outbreak Investigations in 2017 and 2019: A Focus on Traceback, Laboratory, and Collaborative Efforts, Journal of Food Protection,, Pages 2002-2019, https://doi.org/10.4315/JFP-21-082.
[5] Cho IH, K. S. (2017). Current Technical Approaches for the Early Detection of Foodborne Pathogens: Challenges and Opportunities. . Int J Mol Sci. , 18(10):2078. doi: 10.3390/ijms18102078. PMID: 28974002; PMCID: PMC5666760.
[6] Di Febo, T. S. (2019). Development of a Capture ELISA for Rapid Detection of Salmonella enterica in Food Samples. . Food Anal. Methods 12, 322–330. https://doi.org/10.1007/s12161-018-1363-2.
[7] Gupta P, A. A. (2022). Novel Approaches to Environmental Monitoring and Control of Listeria monocytogenes in Food Production Facilities. Foods, 11(12):1760. doi: 10.3390/foods11121760. PMID: 35741961; PMCID: PMC9222551.
[8] Juliana De Oliveira Mota, G. B. (2021). Environmental monitoring program to support food microbiological safety and quality in food industries: A scoping review of the research and guidelines, Food Control, https://doi.org/10.1016/j.foodcont.2021.108283.
[9] M. Focker, E. v.-K. (2023). Designing a risk-based monitoring plan for pathogens in food: A review, Food Control, https://doi.org/10.1016/j.foodcont.2022.109319.
[10] Nehra M, K. V. (2022). Current Scenario of Pathogen Detection Techniques in Agro-Food Sector. Biosensors (Basel), 12(7):489. doi: 10.3390/bios12070489. PMID: 35884292; PMCID: PMC9313409.
[11] Sana Mujahid, R. M. (2021). Occurrence of Listeria monocytogenes in Counter-Sliced Turkey Meat Samples from Independent Delis in New York City,. Journal of Food Protection,, Pages 587-591, https://doi.org/10.4315/JFP-20-335.
[12] Valledor S, V. I.-R. (2020). Comparison of several Real-Time PCR Kits versus a Culture-dependent Algorithm to Identify Enteropathogens in Stool Samples. Sci Rep, 10(1):4301. doi: 10.1038/s41598-020-61202-z. PMI.
[13] Zeng D, C. Z. (2016). Advances and Challenges in Viability Detection of Foodborne Pathogens. Front Microbiol., 7:1833. doi: 10.3389/fmicb.2016.01833. PMID: 27920757; PMCID: PMC5118415.