Absolute Antibody has announced the availability of SARS-CoV-2 neutralizing antibodies derived from individuals infected with COVID-19.
The antibodies, originally generated by Fred Hutchinson Cancer Research Center, have been engineered into recombinant formats useful for COVID-19 research and diagnostic development.
They are now available to scientists and diagnostic developers worldwide via Absolute Antibody’s online catalog.
The new antibodies were generated from the blood cells of an infected COVID-19 patient and have shown in a recent article to display neutralizing activity against SARS-CoV-2, the coronavirus that causes COVID-19.
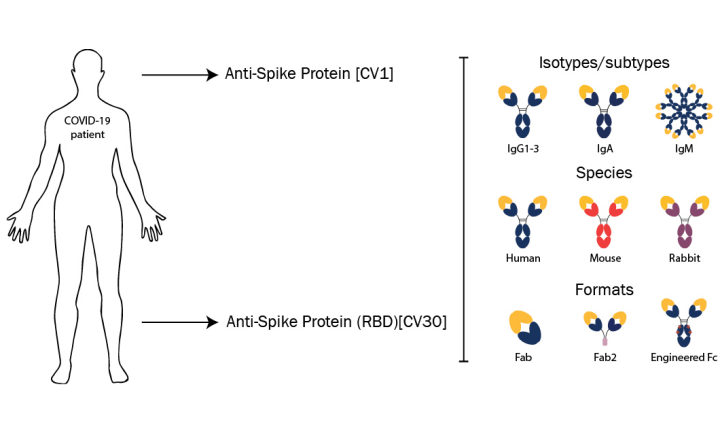
Two antibody clones (CV1 and CV30) each bound the SARS-CoV-2 spike glycoprotein, with CV30 also shown to bind the receptor-binding domain (RBD) and inhibit the interaction with the host cell receptor ACE2.
Both antibodies are now available in a variety of species, isotypes and subtypes designed to extend their usefulness in COVID-19 applications.
Absolute Antibody offers the SARS-CoV-2 antibodies in human formats such as IgG1, IgG3, IgM and IgA, for use in neutralization assays and as serological controls in COVID-19 diagnostic tests.
In addition, the antibodies are available in species such as mouse and rabbit for detection applications and co-labelling studies; fragment formats for better tissue penetration and in vivo imaging; and with engineered Fc Silent™ domains to facilitate research into antibody-dependent enhancement.
All antibodies are recombinantly produced for ensured batch-to-batch reproducibility, high purity and low endotoxin levels.
“By licensing antibodies from the prestigious Fred Hutchinson Cancer Research Center, we can now supply antibodies derived from patients infected with COVID-19, a unique tool that can provide valuable insights into the coronavirus disease and help in designing improved diagnostic tests”, said Amelia Gibson, PhD, MBA, Senior Director of Product Licensing at Absolute Antibody.
The new neutralizing antibodies join Absolute Antibody’s full collection of engineered coronavirus reagents, including the spike glycoprotein antibody clone CR3022, nucleoprotein antibodies, ACE2 Fc fusion proteins, and anti-human immunoglobulin antibodies for use in diagnostic tests.
Absolute Antibody is also supporting coronavirus research by providing antibody engineering and manufacturing services, including the production of gram quantities of human antibodies sequenced from recovering COVID-19 patients. For more information, visit our website here.
Note: This content has been edited by a rapidmicrobiology staff writer for style and content.