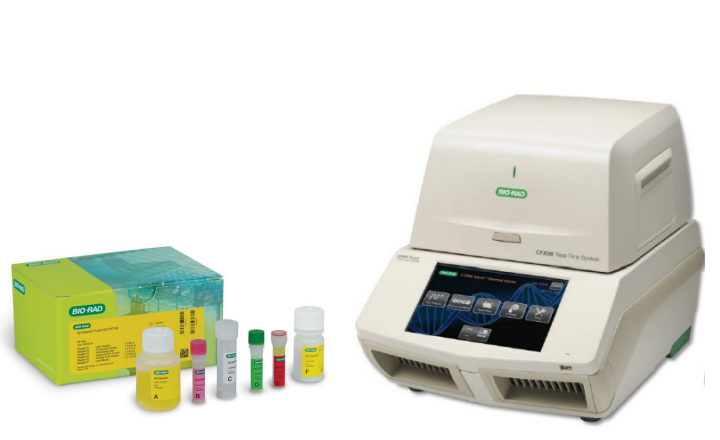
Bio-Rad is pleased to announce that AOAC INTERNATIONAL has evaluated and approved the level 3 modification of the iQ-Check E. coli O157:H7 and iQ-Check STEC VirX and SerO real-time PCR detection kits. Results of the validation study demonstrated no differences between the iQ-Check E. coli O157:H7 (PTM 020801), STEC VirX and SerO (PTM 121203) methods and the reference methods.
The iQ-Check E. coli O157:H7 assay was modified to include 375 g test portions of raw ground beef (83% lean), raw beef trim and fresh spinach, and to include 25 g test portions of raw chicken breast without skin, raw chicken thigh with skin, mechanically separated chicken and raw ground pork. The iQ-Check STEC VirX and SerO assays were modified to include 375 g test portions of raw ground beef, raw beef trim and fresh spinach from buffered peptone water. The modification included the use of the iQ-Check Free DNA Removal Solution.
The modification included a single enrichment in Buffered Peptone Water. This harmonized method allows for the simultaneous enrichment and subsequent detection of Salmonella and pathogenic E. coli from the same sample, providing both a time and cost savings when testing large sample sizes.
The iQ-Check kits for foodborne pathogen detection are routinely used in food safety programs worldwide and are recognized by several renowned international validation organizations.
For more information on Bio-Rad’s complete range of iQ-Check real-time PCR test kits, visit
www.bio-rad.com/iqcheck