- ISO-validated and AFNOR-certified
- Detect both Salmonella and Cronobacter in a single test
- User friendly workflow
- Rapid results
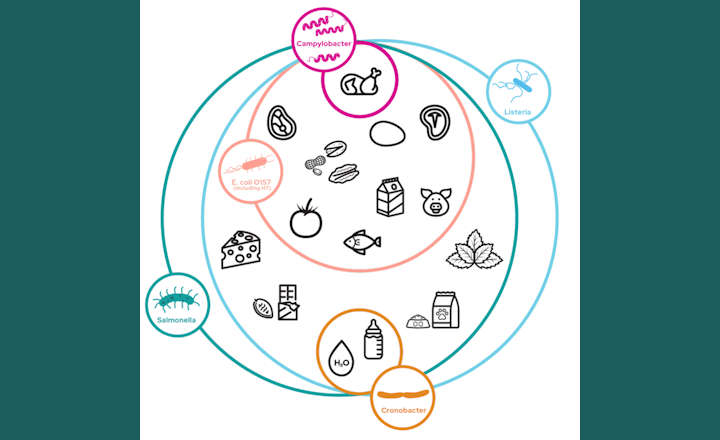
Powdered infant formula is highly susceptible to microbial contamination due to its complex ingredient formulations, low-moisture properties, and how it is manufactured. The presence of microbial contaminants in infant formula poses an increased risk because of the vulnerability of the intended consumer population. Pathogens such as Cronobacter sakazakii and Salmonella are among the most concerning, as both have been linked to severe illness and fatal foodborne infections in infants. Clostridium botulinum is another pathogen of concern that recently gained attention following an infant formula recall in the United States linked to infant botulism cases. Controlling these contaminants requires rigorous safety measures, including hygienic processing standards, robust process monitoring and comprehensive in-process sampling strategies that include real-time microbial monitoring at critical control points and in the finished product.
In fact, infant formula producers must ensure products are free of microbial contamination. Regulatory agencies, including the EFSA, FSANZ, SAMR and FDA, require that infant formula undergo pathogenic screening for Salmonella and Cronobacter. Furthermore, ISO has published standards for Salmonella, Cronobacter, and Enterobacteriaceae testing to ensure infant formula safety. However, these traditional testing methods require separate tests for each pathogen, increasing time, cost, and complexity.
Hygiena® is revolutionizing food safety with the foodproof® Salmonella plus Cronobacter Detection LyoKit® PCR assay, the first and only ISO-validated solution that allows manufacturers to detect both Salmonella and Cronobacter in a single test. This breakthrough simplifies workflows, accelerates results, and enhances operational efficiency for powder infant formula producers and ingredient suppliers.
Manufacturers can now prepare one sample and obtain both Salmonella and Cronobacter results simultaneously. Furthermore, the assay is ISO-validated and AFNOR-certified, ensuring high accuracy and compliance with international food safety standards. In addition, the assay provides results in just 19 hours, allowing manufacturers to release products faster than ever with confidence in reliable results. The user-friendly workflow simplifies testing with lyophilized chemistry for stability, streamlines hands-on steps, and seamlessly integrates with the BAX® System Q7 for easy result interpretation. In addition, we offer PCR solutions for the detection of Clostridium botulinum and other pathogens of concern.
Learn how our solution can help you meet regulatory requirements with confidence.