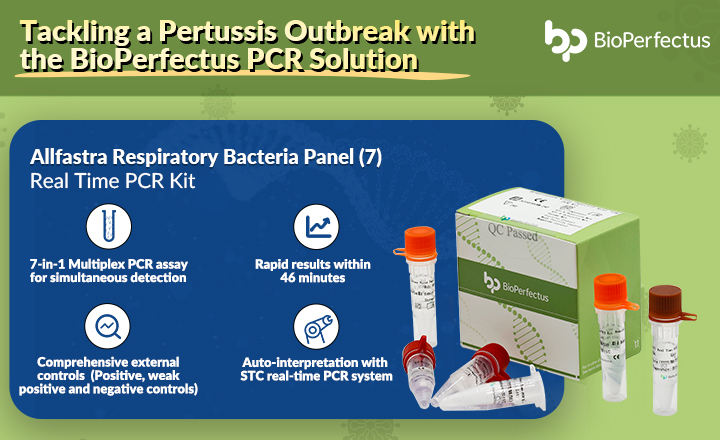
Bioperfectus Technologies Influenza A And B Viruses Real-Time PCR Kit is an in vitro diagnostic test, based on real-time PCR technology, for the detection of Influenza A and B Viruses RNA. Samples can be obtained from throat swab, nasal and pharyngeal secretions and cell culture supernatant.
Key Points:
- Target region: Influenza A and Influenza B specific RNA
- Clinical Sensitivity: 96.1% (Flu A) 97.5% (Flu B)
- Clinical Specificity: 98.7% (Flu A) 98.2% (Flu B)
- Limit of detection: 2.0 TCID50/mL
- Sample input volume: 5μL
- Sample type: RNA in clinical specimens, including throat swab, nasal and pharyngeal secretions and cell culture supernatant