Control of drug-resistant organisms is a national problem. Pathogens are acquiring resistances and developing novel ways of surmounting antimicrobials at unprecedented rates.
Drug discovery has dropped precipitously, and these organisms are nearly ahead of the curve.1 In some instances, orders of bacteria, such as carbapenem-resistant Enterobacterales are impenetrable to most available drugs, limiting treatment options drastically.
Disinfection and sterilization in hospitals is now a focus of concern, as these infections are most pervasive in such settings. Transmission of healthcare-acquired pathogens (HAP) is chiefly related to the contamination of surfaces and equipment, including medical devices (i.e. catheters and ventilators).2,7
Nosocomial, or hospital-associated infections (HAI), are a leading cause of morbidity and mortality in the United States; each year, about 1 in 25 U.S hospital admissions are diagnosed with at least one infection because of complications in healthcare.3,4
HAIs are characterized as infections that occur while receiving healthcare and were otherwise absent or incubating at the time of admission.5
These types of infections are scrupulously monitored by the National Healthcare Safety Network (NHSN) of the Center for Disease Control and Prevention (CDC) and are a top priority for the U.S. Department of Health and Human Services (HHS).5,6
The CDC classifies HAIs as “Winnable Battles”, and commits to this level of surveillance to reduce the incidence of HAIs and improve patient outcome.4,5,8
HAIs include central line-associated bloodstream infections (CLABSI), catheter-associated urinary tract infections (CAUTI), surgical site infections (SSI), Hospital-acquired Pneumonia (HAP), and Ventilator-associated Pneumonia (VAP).5,7
While there are many diseases and organisms in healthcare settings, the CDC regards the emergence of Carbapenem-Resistant Enterobacterales (CRE) as an urgent threat, requiring immediate action.9
CRE are a serious threat to public health. Mortality rates of up to 50% have been associated with hospitalized patients.10
According to the CDC’s 2019 Antibiotic Resistance (AR) Threat Report, U.S CRE cases are stable. This observation speaks to the success of the currently implemented infection control and other preventive strategies; however, continued action is needed.11,12
CDC’s AR initiative, dubbed ‘Detect and Protect,’ heavily relies on the institution of surveillance programs. The lack of preemptive screening of patients in healthcare facilities has contributed to the preventable transmission and spread of CRE.13,14.
Carbapenem resistance among Enterobacterales can result from several different resistance mechanisms; however, resistance through the production of carbapenemases is of utmost concern.
Carbapenems are generally administered as a last resort for treating drug-resistant Gram-negative infections.15
Approximately 30% of all CRE carries a mobile genetic element (MGE) that codes for carbapenemase production, which can facilitate the transfer of resistance. 9,12
While there are many variants of these enzymes, most carbapenemase producers harbor one or more of the ‘big five’ families: KPC, OXA-48-like, NDM, VIM, and IMP. Diagnostic laboratories should therefore implement assays that detect at least four, or preferably, all five of these families.18
Enzyme detection is critical to effective treatment, since it directs the course of antibiotic therapy, and thus promotes Antimicrobial Stewardship.
Antimicrobial Stewardship refers to a collection of efforts that promotes the appropriate use of antimicrobials aimed at improving patient outcomes, reducing AMR, and decreasing the spread of multidrug-resistant organisms.16
More than 2.8 million antibiotic-resistance infections occur in the U.S each year, and more than 35,000 people die as a result.12
In 1945, Alexander Fleming, the discoverer of penicillin (1928), had a premonition of antibiotic-resistant infections; he warned that doctors were abusing penicillin by prescribing it to those who did not need it.
He said: “In such cases, the thoughtless person playing with penicillin is morally responsible for the death of the man who finally succumbs to infection with the penicillin-resistant organism. I hope this evil can be averted."17
To help its customers fight back against AMR, Hardy Diagnostics offers tools to implement the most robust infection control measures.
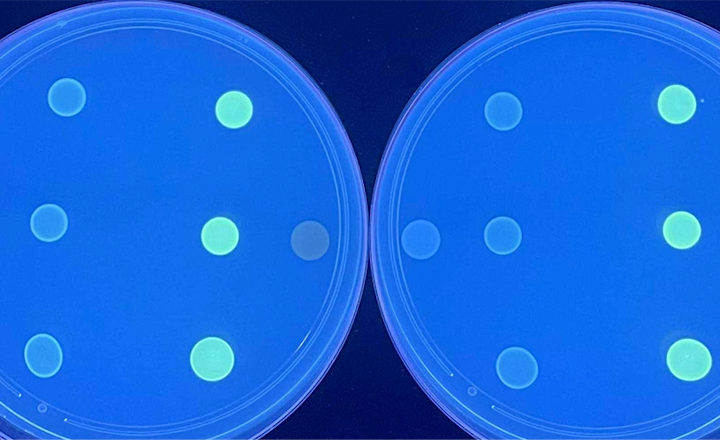
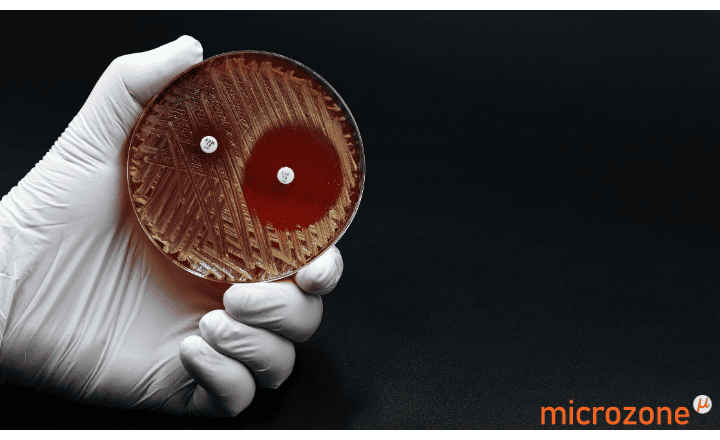
These rapid and convenient methods screen suspect CRE* isolates with HardyCHROM CRE and then confirm and identify the major carbapenemase enzymes with the phenotypic NG-Test® CARBA 5 kit. This kit produces results in only 15 minutes and requires no equipment.
* HardyCHROM CRE for the screening of E. coli, K. pneumoniae, K. aerogenes, K. oxytoca, Enterobacter cloacae complex (ECC), and Serratia marcescens.
References:
- 'Race against time to develop new antibiotics'.
- 'Options for Evaluating Environmental Cleaning'.(download)
- 'Nosocomial or Hospital-acquired Infections: An Overview'.
- CDC Winnable Battles Final Report.
- Hospital Acquired Infections.
- Preventing Healthcare-associated infections.
- Types of healthcare-associated infections.
- CDC: What is a Winnable Battle?
- Carbapenem-Resistant Enterobacteriaceae (Factsheet for download)
- Healthcare Facilities: Information about CRE.
- 'Key Takeaways From the U.S. CDC’s 2019 Antibiotic Resistance Threats Report for Frontline Providers'.
- Antibiotic Threats in the United States (2019)
- 'Screening for carbapenem-resistant Enterobacteriaceae: Who, When, and How?'.
- Detect and Protect Against Antibiotic Resistance. (download)
- Association between antibiotic consumption and the rate of carbapenem-resistant Gram-negative bacteria from China based on 153 tertiary hospitals data in 2014.
- Antimicrobial stewardship.
- How Can We Turn the Tide Against Inappropriate Antibiotic Prescriptions?
- Introducing NG-Test® CARBA 5.