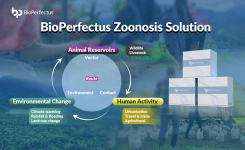
-
Home
- Featured Products
- Page no.: 18
Featured Products
INVALID PAGE NUMBER. Please choose page below.
LATEST MICROBIOLOGY NEWS
MICROBIOLOGY EVENTS
-
4th International Beverages Quality Conference 2026
3 Mar 2026 -
Cereulide: What the Food Industry Needs to Know Now
5 Mar 2026 -
Forum LABO 2026
10 Mar 2026 -
2026 QC Micro Summit
17 Mar 2026 -
Advanced Therapies
17 Mar 2026 -
ISPE Aseptic Conference 2026
23 Mar 2026 -
Analytica 2026
24 Mar 2026 -
GMP PharmaCongress and GMP PharmaTechnica Expo 2026
24 Mar 2026 -
ESCMID Global 2026
17 Apr 2026 -
Essentials Of USP Microbiology - Reading Between the Lines of the USP General
28 Apr 2026