Rapid Culture Techniques for Manual Microbiology - the Next Generation
Key Points
- Techniques that rely on organism growth and metabolism to produce a characteristic reaction
- Novel media contains specific growth promoters, inhibitors and/or chromogens
- Easy to validate, low cost per test
 Microbiology is on a cusp; on one side, for almost twenty years the word rapid has been applied to developments in methodology reliant upon growth-based, cultural techniques. On the other side, real-time polymerase chain reaction (RT-PCR) assays can now, in some situations, offer same day results and are potentially poised to turn the microbiology lab into the molecular diagnostics department.
Microbiology is on a cusp; on one side, for almost twenty years the word rapid has been applied to developments in methodology reliant upon growth-based, cultural techniques. On the other side, real-time polymerase chain reaction (RT-PCR) assays can now, in some situations, offer same day results and are potentially poised to turn the microbiology lab into the molecular diagnostics department.
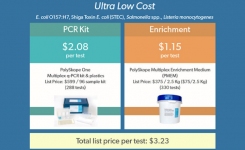
However such techniques normally come at an 'added value' price that many labs either cannot afford or cannot justify, if for example there is no definable cost benefit to the organisation in real time results. These labs require tests that are low capital and lower consumable costs, easy to establish and validate, with low maintainance and minimal technician training required.
So there is still very definately a need for novel cultural technologies designed to take days off the standard time to result. Let’s examine what’s on offer for rapid manual cultural microbiology - this may be the last stop before PCRville but they still offer many advantages.
Technology
At a practical level, microbiology can be split between pathogen testing and those organisms that will cause product spoilage and/or stability issues. It is also possible to draw a line between true cultural techniques and those using some metabolite to indicate that growth has occured normally by adapting the presentation of the media to include devices other than Petri dishes, and invariably using an instrument of some description, here we are just going to focus on novel cultural media.
As a general rule, with the exception of fungal tests, most quality indicator organisms can be reported within 48 hours utilizing traditional media inoculated direct from a homogonized sample. These media formulations have been relatively unchanged for at least the last twenty-five years with efficacy largely empirically derived.
In terms of time to result, the same cannot be said for pathogens. With the requirement for more sensitive detection limits, an enrichment stage, or stages, needs to be included followed by confirmatory tests. This not only extends the time to result, (and this can be up to seven days for Salmonella spp and Listeria spp), but also increases labor and consumable costs. With commercial pressures acting to reduce the reporting timeframe, traditional growth media formulations began to evolve.
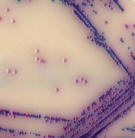
Leading this change away from traditional, empirically derived selective and diagnostic features were the chromogenic and fluorogenic plating media. Such media allowed species specific diagnostic features to be designed into direct plating media that would previously have required sub-culture and further testing. An early example would be the inclusion of the fluorogenic substrate methylumbelliferone -β -D-glucoronide (MUG) into bile salt media for the specific diagnosis of E.coli amongst lactose fermenting, bile tolerant Gram negative flora.
Chromogenic substrates followed closely, allowing colour to report the presence of specific species amongst closely related accompanying flora. Early examples were designed to report extra-cellular enzymatic activity and as such the reactions were not colony associated and diffusion of the colour into the surrounding media occurred. As with all emerging technologies there were some early problems and the regulatory framework was slow to adopt the novel media. However the take-home message for microbiologists was clear; cultural techniques could now be used to reduce extended reporting timescales by making species specific diagnostics available on primary plating media.
The latest generation of chromogenic media take this even further. From an improved understanding of genotype and gene expression, specific intra-cellular enzyme systems are targeted with substrates that produce colony associated colour reactions. Also, combining enzyme/substrate systems within the same medium allows differential diagnosis to take place, e.g. UTI media that can report the presence of 2 or 3 target groups of organisms on one plate.
The very latest innovations in rapid cultural techniques reduce the time taken to enrich target pathogens in recovery systems designed to lead into chromogenic diagnostic media. With the inclusion of an inhibitor it is now possible to specifically reduce the growth rate of a competitor species within a recovery system. If the recovery system also contains specific growth promoters for the target species then a single broth can combine a resuscitation and growth stage together into one enrichment. This is a revolution for the food lab, cutting two steps down to one, reducing labor costs, and allowing earlier inoculation onto specifically designed chromogenic plating media.
Such systems designed for Salmonella spp and Listeria spp allow diagnostic recovery within 48 hours as opposed to a more typical 5 day test. These cultural techniques have addressed the commercial pressures to report earlier on the microbiological status of valuable inventory, without the need to invest in capital equipment. Regulatory acceptance of these novel approaches is well advanced with ISO standards now incorporating single broth enrichment followed by chromogenic plating.
Benefits
Single broths can combine resuscitation and growth stages reducing labor and consumables
Chromogenic plating offers easy identification and differentiation reducing the number of presumptives
Start-up costs are significantly lower than instrumental techniques
Improved regulatory recognition makes them more accessible for SME organizations.
Get the latest updates in Rapid Microbiological Test Methods sent to your email?
Subscribe to the free rapidmicrobiology eNewsletter