The Toximaster® QC8 software supports data processing based on the protocols complying with three types of pharmacopeia (USP/EP/JP) for bacterial endotoxin testing. This software is compliant with FDA 21 CFR Part 11 ERES (electronic records/electronic signature), which requires the proper information from all parties involved in the testing of the sample. Toximaster® QC8 offers excellent audit trail capabilities, can provide statistical processing of means and standard deviations, and has robust data processing functions. These data processing functions can be performed by defining sample types such as standards, controls, and test samples.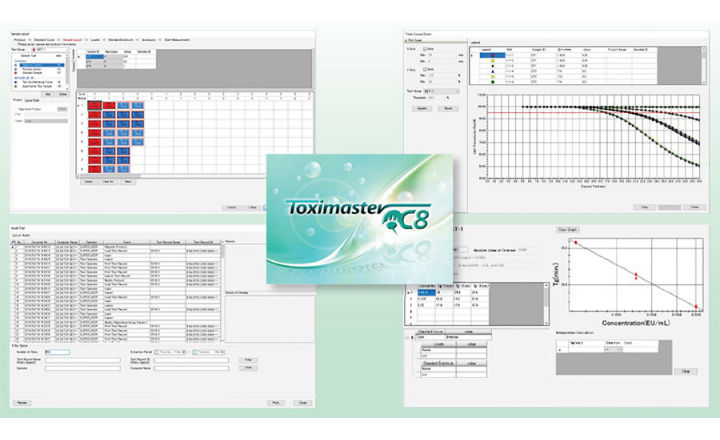
Key Points:
- FDA 21 CFR Part 11 ERES compliant
- Endotoxin determination in compliance with pharmacopeias (USP/EP/JP) for BET
- Capable of creating three types of curves: internal standard curve, manual input curve and a measured curve
- Product endotoxin limit and MVD generation
- Allows trending of product results over time and early detection of potential product failures
- Generates hard copy printouts with all pertinent information for routine audits through three unique audit trails
- Creation of a standard workflow to be reviewed, confirmed and approved prior to operating
- Security functions to lock the application, disable an account and lock out the system
- Backup of the user management database and automatic backup of the system information database
- Improved precision and accuracy over the traditional gel clot method
- Ideal software for comparative testing and validating most LAL
To find out more use the green "request more information" button below.