Selux Diagnostics Inc., has been given the green light by the US Department of Health and Human Services (HHS) to progress its same-day AST platform.
Last year, Selux Diagnostics (SD) received $9 million upon entering into a 5-year contract worth $45 million with BARDA (Biomedical Advanced Research and Development Authority), the research arm of the HHS. However, the remaining monies only become available if SD reach the targets set by BARDA. Last week, SD announced they had reached their first target three months in advance and duly received $11.4 million for their efforts.
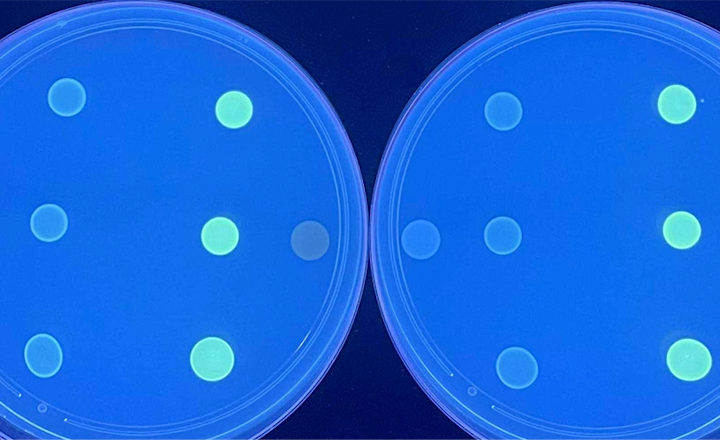
The first part of the contract was to advance clinical trials for SD’s Next Generation Phenotyping platform (NGP). This technology identifies bacteria based on observable physical or biochemical characteristics of the bacteria. Based on the characteristics the test identifies the class of bacteria and which antibiotics are best for treating that particular infection. The proprietary Selux technology promises to provide results 2-5 times faster and for 3-4 times the number of antibiotics than current systems can.
The second- part of the contract, which applies to the recent monies transferred to SD, is to advance on their 2nd generation rapid sepsis diagnostic platform which promises to give same-day results.
“The hunt continues for a rapid, broad-menu, cost-effective NGP platform that brings AST into the 21st century. Results should be available within five to six hours to enable the same-shift reporting necessary to keep pace with rapid ID technologies and eliminate the ID-to-AST gap” said Eric Stern, Co-Founder of Selux Diagnostics.
Eric Stern met fellow SD Co-Founder Aleksander Vacic at Yale University and set up SD five years ago in an improvised attic laboratory. Since then they have raised $25 million from Sands Capital Ventures on top of the BARDA contract to support their NGP platform in its application for FDA clearance.
Note: This content has been edited by a rapidmicrobiology staff writer for style and content.