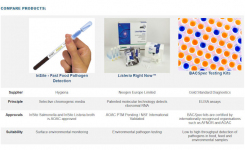
The microbiological quality control testing of biotherapeutics such as cell and gene therapy products can present some unique issues for manufacturers.
With complex raw materials and low production volumes even getting sufficient product to test is a challenge in itself, and with such high-value products both in terms of patient outcomes and production costs, every drop counts!
Plus, when working with some extremely short shelf lives, sometimes just measured in hours, waiting for traditional compendial testing is not an option. Here our rapidmicrobiology special focus explore some solutions for your microbial testing of biotherapeutics.
Rapid Microbial Methods for Short Shelf-Life and ATMP Testing
The Celsis Adapt™ concentrator, in combination with Celsis Adapt™ Cell Kit and Celsis® luminometers, unlocks a rapid microbiological contamination detection platform for multiple quality control testing of short shelf-life products, ATMPs, cell, and gene therapy production.
Find Out More
SterilGARD® e3 BioPharma Package Ideal for Cell and Gene Therapy and Personalized Medicine.
Meeting the compliance demands in biomanufacturing environments can be challenging. Baker offers a variety of products and services, like the SterilGARD® e3, to help support your quality management.
Find Out More
Advantages of Ready-to-Use Culture Media for Mycoplasmas. Now with Extended Shelf Life!
How ready-to-use liquid and solid culture media with a longer shelf life can help to make Mycoplasma testing of cell cultures, raw materials, intermediates, and final products according to EP 2.6.7 & USP 63 easier.
Find Out More
Strengthen Cell and Gene Therapy Production with Smart Quality Testing
bioMérieux’s simple, fast, and accurate detection solutions allow labs to test for contamination at every stage of the production process, giving you confidence the therapy the patient receives will be as safe as possible.
Find Out More
How SmartControl EM will Solve your Environmental Monitoring Challenges
Environmental monitoring is a crucial yet challenging task, made more difficult with piles of paper, slow spreadsheets and tough to meet regulation. If this sounds familiar, SmartControl EM can help.
Find Out More
PYROSTAR™ ES-F Reagents are a One-stop Solution for Endotoxin Testing
Endotoxin testing is vital to the production of pharmaceuticals. PYROSTAR™ ES-F reagents provide an endotoxin test that is accurate, specific, and versatile. These features raise confidence in performing an endotoxin test.
Find Out More
INTEGRA Offers Answers to Your Cell Culture Throughput Needs
Increase the throughput of your cell culture workflows by taking advantage of the extensive range of multichannel pipettes available from INTEGRA Biosciences.
Find Out More
Reliability in your Microbiological QC for All Biomanufacturing Steps
Quality Control in biopharmaceuticals is key to ensure the quality and safety of a product. Condalab has available the required culture media and other reagents to perform without complications MFT, Sterility Testing, and Non-Sterile products Testing.
Find Out More
Promicol PRENOVA® Platform – Rapid QC for Vaccine and Cell and Gene Therapy Products
Promicol has developed a method for rapid and reliable QC of vaccine preparations shortening the time to release to 72 hours with Promicol PRENOVA® COV1. The ATP-Bioluminescence-based technology can be applied to the quality control procedures for all kinds of vaccines.
Find Out More
Coriolis Pharma Opens New ATMP Formulation Development Facilities
Coriolis’ new ATMP facilities will meet the growing demand for formulation development and analytical characterization for ATMPs up to BSL2/S2 level and increase Coriolis’ capabilities significantly.
Find Out More
Germfree Launches Novel Mobile Cleanroom Technology for Advanced Therapies
The bioGO is a rapidly deployable cleanroom using standard, proven cGMP facility designs that are pre-commissioned and pre-qualified. The fast, flexible space allows clinical trials to start when needed, without traditional capital building expenditures.
Find Out More
How to Validate and Implement Your Rapid Microbiological Method - Interview With Dr. Michael Miller
Dr. Michael J. Miller, one of the leading global experts on rapid microbiology method validation and implementation, talks to rapidmicrobiology.com about the current status of rapid methods within the pharmaceutical and biopharmaceutical industries and how ATMPs is accelerating adoption.
Find Out More
Testing Biotherapeutics for Mycoplasmas - the Risks and International Regulations
An in-depth Interview with Helena Windsor of Mycoplasma Experience, the only UK company specialising in Mycoplasmology. Now, in their 4th decade of mycoplasma testing, Helena talks about the fast-moving Cell and Gene therapy market and how mycoplasma testing is vital at this critical juncture.
Find Out More