Podcast Summary
COVID-19 makes this one of the more interesting times to introduce our new podcast, which is why we decided to focus it on a critical topic: How to maintain operational efficiencies in your QC lab during an unprecedented time.
Joining us for the discussion were Susan Schilling, our Director of Customer Relations, and David Jones, Director of Technical Marketing and Government Affairs at Rapid Micro Biosystems. They began by explaining the differences between manual, automated, and compendial microbiology methods and discussing which types of pharmaceutical companies are using automated methods most often to maintain production during the pandemic.
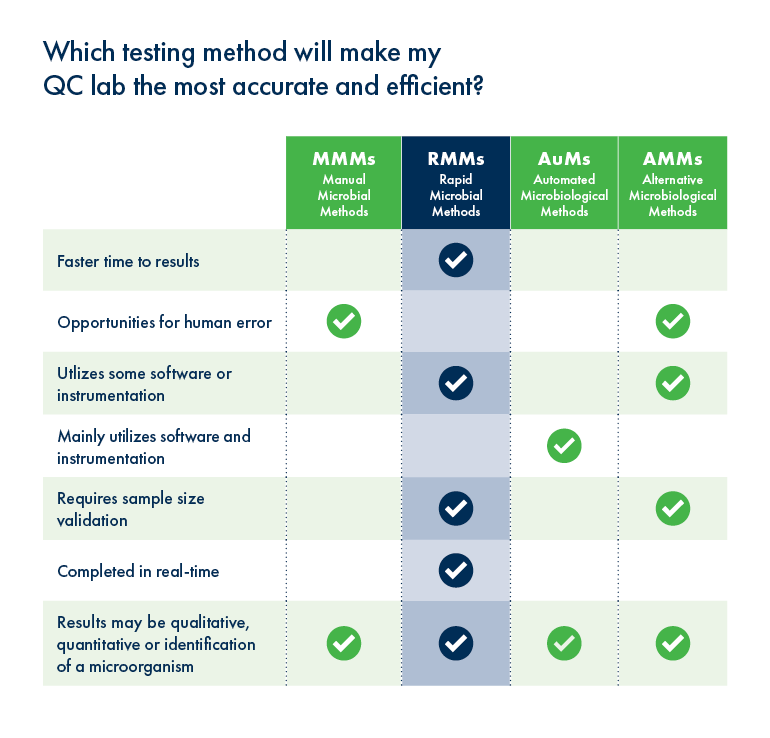
So, which microbial method is best?
Not surprisingly, Susan said that those manufacturing drugs in a shortage state, or that help in intubation or removing intubation, are more likely to be using rapid automation methods right now, simply because they need to release products much more quickly. She also noted that CDMOs who need to perform faster bioburden tests to produce more product are also relying on automated methods. As an example, she says that one Growth Direct customer was able to reduce his company’s bioburden testing time down to as little as 48 hours, which allowed them to release raw materials and APIs that were going to be stockpiled so that they could build a large inventory of the final product and ship those APIs more quickly to their fill and finish facility.
We wondered how the workflow of Growth Direct is helping QC teams manage their pandemic response.
Both Susan and David agreed that the ability to run a virtually hands-free operation was a huge benefit as social distancing continues to be critical for worker safety. Growth Direct makes this possible because once the samples are taken, all of the other steps in the process are automated. David also pointed out that Growth Direct has a remote monitoring system, so technicians or engineers no longer need to be onsite to troubleshoot or manipulate the machine. He added that remote monitoring on an international scale is not just possible but currently necessary for some systems, and RMB has been innovative enough to ensure that those activities continue without interruption.
If your QC micro lab is considering new operational efficiencies, we hope this podcast conversation helped you better understand the benefits of automation.
Looking to learn more?
The impact of COVID-19 has been felt by everyone. Those business teams that are learning from experience, taking time to consider and implement new processes and innovations stand to benefit the most.