The Tasty Tech That ...
INTEGRA’s Elec...
10th February 2021 Product update: rapidmicrobiology staff writer
Increase in PoC AMR Diagnostic for N. Gonorrhoeae Due to Limited STI Testing Supplies
Biomed Diagnostics has increased production of the InTray GC® due to the shortages in STI testing supplies.
The rapid rise in COVID testing, along with projected volume of tests needed monthly, has depleted supplies for other infectious disease testing, primarily sexually transmitted infections. Biomed Diagnostics is responding with a significant increase in production of the InTray GC and the InPouch TV®.
The CDC recommends culture testing for Neisseria gonorrhoeae in tandem with NAAT testing, due to the dramatic increase in AMR strains of the pathogen and state that N. gonorrhoeae poses one of the most urgent threats of antibiotic-resistant infections in the United States and the world. Now, as COVID is ravaging the health care infrastructure, it is also depleting testing supplies used in STI screening.
The emergence of N. Gonorrhoeae strains resistant to previously effective antibiotics sounds an alarm throughout the health care community. There is only one class of antibiotics that are currently deemed an effective treatment for this devastating disease.
A 2021 study published by JAMA Pediatrics recommends universal screening for gonococcal infections in adolescents and young adults who used the emergency room for acute care. The rate of N. gonorrhoeae infections is increasing dramatically in the age group 18-22.
According to a study in The Lancet, up to two-thirds of N. gonorrhoeae infections in women who have oropharyngeal infections, are missed.
For over 10 years, Biomed Diagnostic's has partnered with STD clinics and health care agencies worldwide in the fight against N. gonorrhoeae and antimicrobial resistance (AMR).
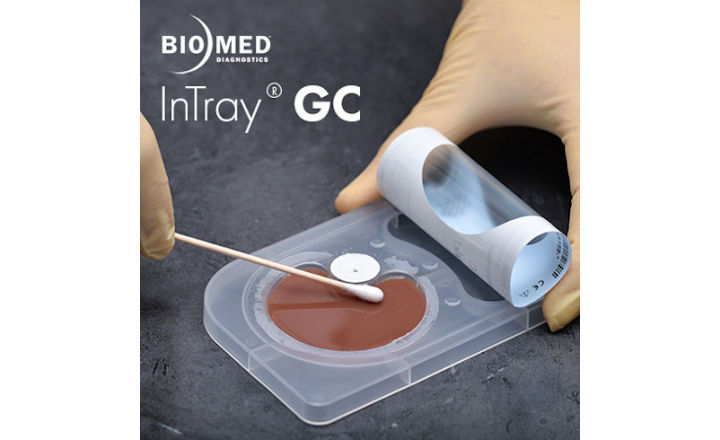
Biomed's diagnostic test - the InTray GC® - is a prepared selective culture for the qualitative detection of oral, rectal and genito-urethral Neisseria gonorrhoeae. It is an essential tool for AMR diagnostics.
The InTray GC is specifically designed for ease-of-use at the point of care and provides visual detection of the microbe.
The media is highly selective and specific to N. gonorrhoeae. The InTray GC, with an embedded C02 tablet, does not require an expensive CO2 incubator, and there are no requirements for replating of the culture for detection. Inoculate the InTray, seal, and observe for microbial growth and color change within 24 hours.
For more information about the InTray GC, please click here.
Tags:
Date Published: 10th February 2021
Source article link: View
Note: This content has been edited by a rapidmicrobiology staff writer for style and content.
The Tasty Tech That Ensures
INTEGRA’s Electronic Pipettes