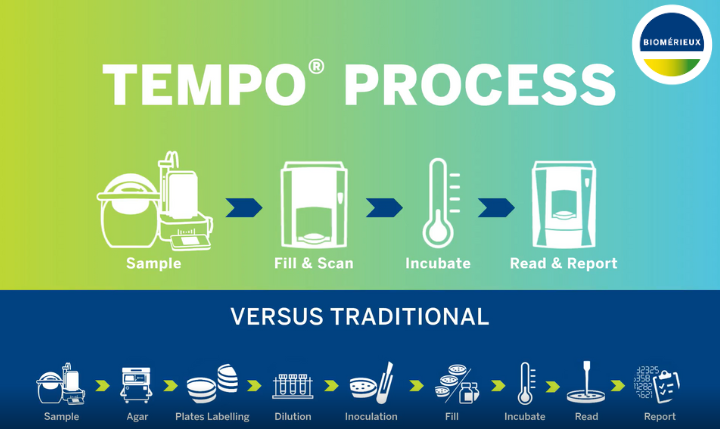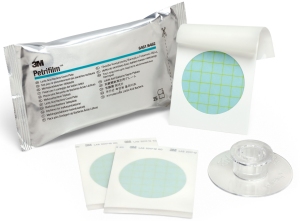
The 3M™ Petrifilm™ Lactic Acid Bacteria Count Plate has been awarded AOAC® Performance-Tested MethodSM, Certificate #041701 for a variety of foods. With this AOAC Performance-Tested Method designation, the 3M Petrifilm Lactic Acid Bacteria Count Plate becomes the first commercial method of its kind to receive a validation from a third-party scientific organization.
The ready-to-use plate, introduced in August 2016, simplifies the testing process for lactic acid bacteria spoilage organisms by serving as a unique all-in-one solution. Companies can use the technology to obtain accurate results in less time, extend shelf life, reduce waste, minimize recalls and improve the look, taste, texture and smell of their products.
The AOAC Performance-Tested Method certification consisted of an independent laboratory validation of the method according to a protocol approved by the AOAC Research Institute. 3M’s plate was tested on an environmental surface and a variety of food matrices as part of the validation, as lactic acid bacteria is a concern for manufacturers of foods including meat, fish, poultry, processed foods, produce, dairy products, dressings and sauces.
Testing allows companies to assess specific lactic acid bacteria levels that are acceptable for their foods, giving them the opportunity to adjust their processing conditions or intensify cleaning and sanitation procedures before releasing product.
This new lactic acid bacteria method eliminates the need for labor intensive media preparation, special diluents and sample pH adjustment needed in traditional lactic acid bacteria testing.
3M Petrifilm Plates are also a more sustainable indicator test solution, producing 66 percent less waste (by weight and volume) compared to competitive agar methods.
For more information, visit product page for the 3M Petrifilm Lactic Acid Bacteria Count Plates here: bit.ly/2fFAzAo.