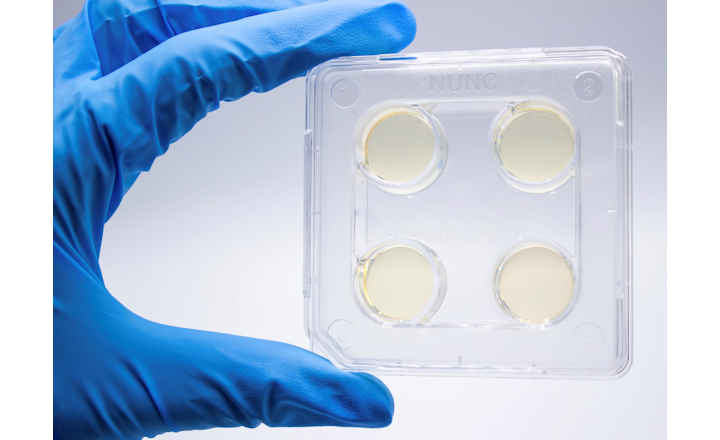
Oritavancin represents one of the latest treatment options for skin infections and is now available on FDA-cleared microbroth dilution susceptibility plates, giving microbiology laboratories the means for susceptibility testing of fastidious and non-fastidious gram positive organisms that cause skin infections in adults.
Recognized for offering one of the largest and most up-to-date selections of FDA-cleared antimicrobials for susceptibility testing, the Thermo Scientific Sensititre ID/AST System is now the first to offer oritavancin on IVD-labeled microbroth dilution susceptibility plates. Oritavancin is currently available in the U.S. on Sensititre custom plates, and will be featured on additional standard plates in the future.
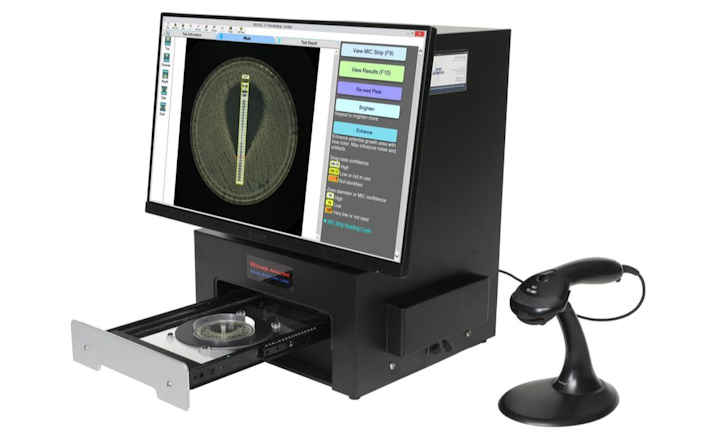
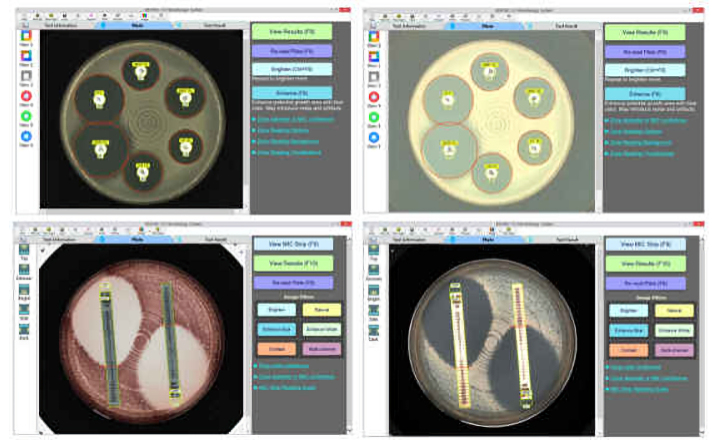
Testing can be performed manually, as well as using the Sensititre Vizion Digital MIC Viewing System, Sensititre OptiRead Automated Fluorometric Plate Reading System, or the fully automated Sensititre ARIS 2X System.
The Sensititre System utilizes true minimum inhibitory concentration (MIC) results, which are essential to fighting antimicrobial resistance, providing greater sensitivity for better resistance tracking. True MIC results are also the best measure of antibacterial effect, which can assist with therapeutic choices, and promote overall better patient care.