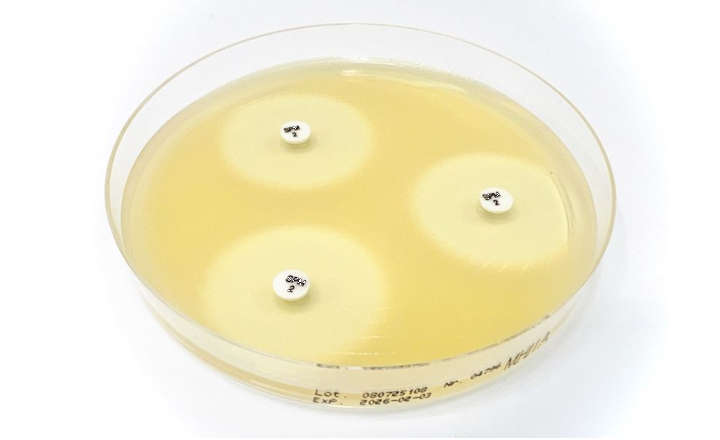
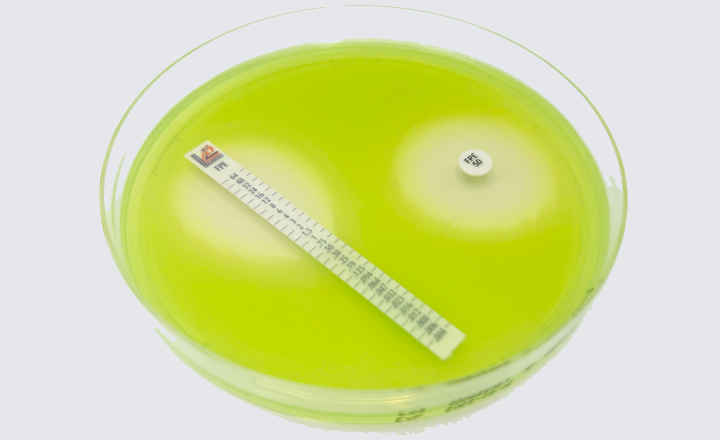
Visit Thermo Fisher Scientific at Booth 1-16 to learn how to gain access to antimicrobial susceptibility testing (AST) results from positive blood cultures 1-2 days faster than traditional methods with the new Q-linea ASTar System, supporting expedited treatment intervention and improved patient outcomes. Designed to move your laboratory forward, the ASTar® System delivers accurate minimum inhibitory concentration (MIC) results in ≈ 6 hours versus up to 72 hours with traditional methods.
Developed by Q-linea and distributed exclusively by Thermo Fisher Scientific, the ASTar System:- Delivers fast, accurate results in ≈ 6 hours direct from positive blood cultures.
- Offers a broad AST panel with a disc design containing more than 300 wells, and report-out from the broadest combination of antimicrobials and dilution ranges in a single test for Gram-negative bacteria*.
- Integrates broth microdilution with imaging technology to deliver a controlled inoculum that maintains the reliability, accuracy and enhanced decision-making benefits delivered by MIC results.
With the ASTar System, you can gain access to results 1-2 days faster than traditional methods to enable expedited treatment intervention and improved patient outcomes. Visit us to see the ASTar System in action!
*Based on commercially available systems market overview undertaken by Q-linea - May 2021
Product is CE marked but not 510(k)-cleared and not available for sale in the U.S. Availability of product in each country depends on local regulatory marketing authorization status. Q-linea AB is the legal manufacturer of ASTar®. ASTar® is a trademark of Q-linea AB. The ASTar system is distributed globally by Thermo Fisher Scientific.
Learn more about what's on the booth #1-16 or contact the supplier for details using the green "Request Information" button below.