A Funded Development Project for New Pharmaceutical Product Line
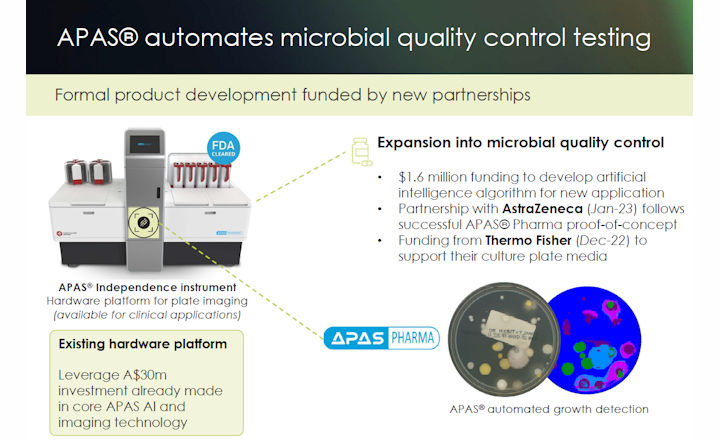
Following a successful proof-of-concept, Clever Culture Systems (CCS) parent company LBT Innovations Limited has been engaged by AstraZeneca, to undertake a full product development project for their new APAS® Pharma analysis module (artificial intelligence software) to be used on the APAS® Independence instrument.
The APAS® Pharma analysis module will be developed to identify microbial growth on settle plates used in sterility monitoring during drug manufacturing.
Under the project, AstraZeneca will fund the development of the APAS® Pharma analysis module and validate the final product for their processes. The project is expected to cost approx. AUD$1m, with payment received based on achieving a series of technical milestones.
The successful APAS® Pharma proof-of-concept was an important achievement that established confidence in the APAS® technology. APAS® Independence is an established hardware platform already developed and used by clinical laboratories. It is expected that an APAS® Independence instrument will be deployed to AstraZeneca to support data collection and testing as part of the development for the new APAS® Pharma analysis module.
CCS CEO and Managing Director, Mr Brent Barnes said:
“This partnership is really exciting for the Company. AstraZeneca leads this field and is looking to innovate its processes and set the standard for others to follow. Our APAS® technology is ideally suited for the application of microbial quality control, able to improve consistency of results and drive standardisation across manufacturing sites.”
Pharmaceutical Microbial Quality Control
Microbial quality control is an important production control process used to monitor the environment during sterile drug manufacture. In this process, settle plates are used continuously for the detection of microbial contamination in the air, with the vast majority, over 90%, of settle plates being negative (i.e., showing no microbial growth).
Interpretation of settle plates is subjective and relies on manual reading and reporting, which is subject to human error. This has led to increasing data integrity requirements from regulators, including the need for independent analyst verification before results can be released.
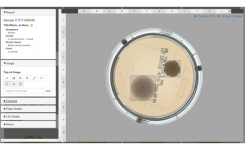
CCS’s APAS® technology is ideally suited to the application, providing improved data integrity through automation and eliminating the subjectivity of manual plate reading. APAS® Pharma uses CCS’s cutting-edge imaging and artificial intelligence algorithms to automatically detect growth on settle plates, creating a digital record and plate image at the time of processing, providing increased traceability of the result. Once validated, APAS® Pharma will automatically report negative results, providing an important time-saving and improved quality control traceability of results to pharmaceutical laboratories.
The Automated Plate Assessment System or APAS® Independence uses CCS's intelligent imaging and machine learning software to automate the imaging, analysis and interpretation of culture plates following incubation. The technology remains the only US FDA-cleared artificial intelligence technology for automated culture plate reading. Themo Fisher Scientific, Inc. is the exclusive distributor of the APAS Independence in the USA and throughout Europe.
Visit cleverculturesystems.com/apas-pharma
Note: This content has been edited by a rapidmicrobiology staff writer for style and content.