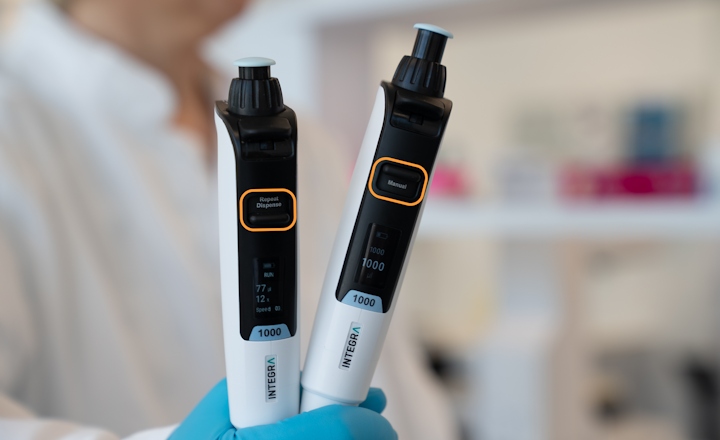
The Cubis® II analytical balance is designed for high-performance weighing and offers digital workflow management following US FDA data integrity principles (ALCOA). On-board QApps contribute to correct usage by guiding through weighing processes, increasing measurement accuracy, precision, and process safety.
The Pharma Software Application Package supports compliance with FDA 21 CFR Part 11 and EU GMP Annex 11, USP Chapter 41, and includes features like user management, digital signatures, audit trail, and minimum sample weight determination
Discover solutions here or use the Request Information button below to contact the supplier.