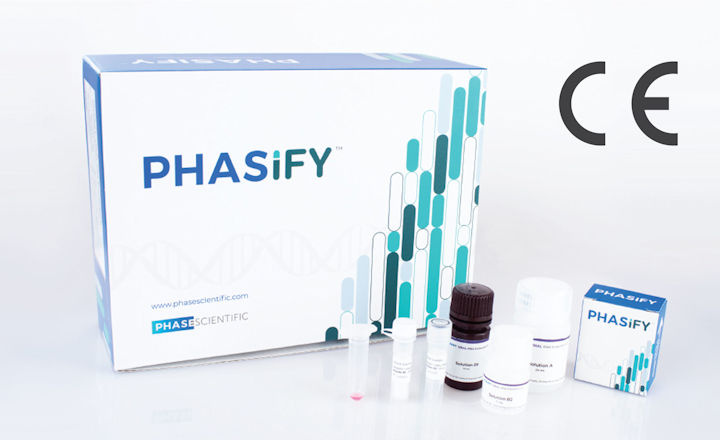
PHASE Scientific has received CE-marking for its PHASIFY™ VIRAL RNA Extraction Kit. The PHASIFY VIRAL RNA Extraction Kit is designed to purify and concentrate viral RNA in patient viral transport media samples.
Attested by the company, PHASIFY VIRAL improves input sample quality, enabling earlier detection, improved overall sensitivity and bolsters confidence in COVID-19 diagnostic test results.
According to Jeffrey D, Klausner, Ph.D., Principal Investigator at the National Institutes of Health (US) and Professor of Medicine and Public Health at UCLA "Utilizing PHASIFY VIRAL RNA extraction can result in a 15-30-fold greater amount of viral RNA for standard RT-qPCR analyses, the increased sample quality can improve analytic sensitivity and reduce false-negative results."
The PHASIFY VIRAL product can facilitate higher SARS-CoV-2 RNA yields. According to the company, in-house and clinical validation data have shown PHASIFY VIRAL can enable a 3-6 cycle threshold reduction compared to solid-phase extraction. Clinical studies have also demonstrated PHASIFY VIRAL increases true positive detection compared to conventional solid-phase extraction.
This capability is realized through PHASE Scientific's proprietary technology PHASIFY, which can isolate and concentrate target molecules, making them easier to detect. Incorporating the technology can make diagnostic tests more affordable, accessible, faster, easier, and more accurate.
Additional advantages of PHASIFY VIRAL include the ability to increase the sample input volume up to 600µL per extraction and enhance final viral RNA concentration with flexible elution volumes down to 10µL. Its easy-to-use design requires no additional specialized equipment such as magnetic racks and vacuum pumps for operation.
PHASE Scientific has developed an array of products to combat COVID-19, which includes rapid antibody tests, sample preparation kits, and RT-qPCR kits.
Note: This content has been edited by a rapidmicrobiology staff writer for style and content.