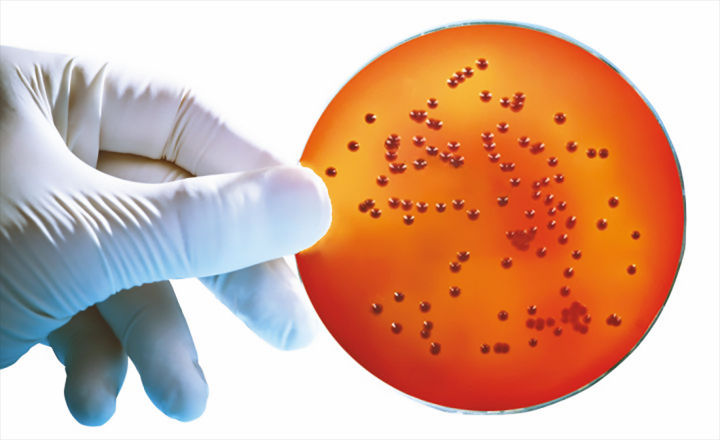
SGL is excited to introduce a pre-poured Burkholderia cepacia Selective Agar (BCSA) in 90mm plate format, a selective growth medium specifically designed to meet the formulation requirements listed in USP <60> to recover Burkholderia cepacia, a bacterial group of rising importance in the pharmaceutical, clinical and cosmetic industries in recent years.
Burkholderia cepacia is a member of a group of at least 20 closely related species in the Burkholderia cepacia complex (BCC). BCC strains are some of the most versatile Gram-negative bacteria with a wide environmental distribution in soil, plants, and water.
The Impact of Burkholderia cepacia on Pharmaceuticals
Healthcare-associated outbreaks of BCC have been linked to contaminated nebulized and intravenous solutions, including commercially distributed products.
In the past decade, organisms that fall within the BCC grouping have been identified as problematic within the pharmaceutical manufacturing environment.
Testing for Burkholderia cepacia
Until recently there was no compendial testing procedure detecting BCC in pharmaceutical components or finished product. On December 1, 2019, The United States Pharmacopeia (USP) published chapter <60> Microbiological Examination of Non-sterile Products - Tests for BCC. The USP <60> specifies the test strains for growth promotion, methodology and selective media recommended to ensure the absence of BCC.
Why Choose SGL Burkholderia cepacia Selective Agar (BCSA)?
- Meets the USP formulation criteria
- Quicker and better isolation of BCC
- Increased selectivity
- Fewer false positives
SGL BCSA pre-prepared media contains numerous nutritional components, and energy sources that support BCC growth, and a mixture of antibiotics designed to prevent the growth of micro-organisms including typical environmental isolates not belonging to BCC such as Pseudomonas and Staphylococcus species.
This allows for easier detection of BCC in test samples containing background contaminants.
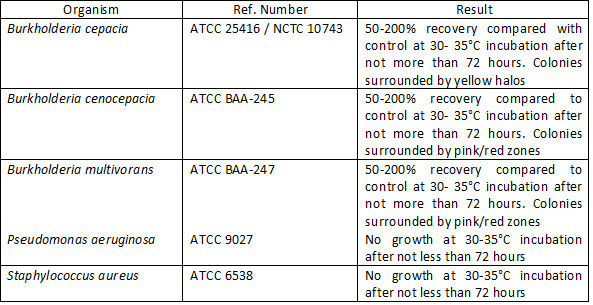
Quality Control
SGL supplies a wide range of other pre-poured media for use in pharmaceutical, cosmetic, clinical, and veterinary applications. These include standard off the shelf media as well as bespoke media products tailored to customer-specific formulations.
Representative samples of our products are QC tested in our on-site laboratory which is UKAS accredited to ISO 17025.
Visit www.sglab.com for details on this and the full pharmaceutical media product range or use the green "Request Information" button below.