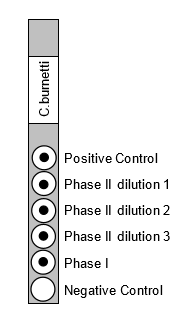
The ImmunoDOT Rickettsia and Coxiella Tests utilize an EIA dot technique for the detection of antibodies. The antigens are dispensed as discrete dots onto a solid membrane. After adding specimen to a reaction vessel, an assay strip is inserted, allowing patient antibodies reactive with the test antigen to bind to the strip’s solid support membrane. In the second stage, the reaction is enhanced by removal of non-specifically bound materials. During the third stage, alkaline phosphatase-conjugated anti-human antibodies are allowed to react with bound patient antibodies. Finally, the strip is transferred to enzyme substrate reagent which reacts with bound alkaline phosphatase to produce an easily seen, distinct dot.
ImmunoDOT kits available for C. burnetti, R. typhi, R. conorii and R. rickettsia. The ImmunoDOT Rickettsia and Coxiella tests are CE marked and Coxiella test is FDA approved.