A new diagnostic test has been made available that will help in the diagnosis and proper treatment of patients at risk for invasive aspergillosis.
Aspergillosis is a rare fungal infection caused by Aspergillus, a species of mould that lives indoors and outdoors all over the world. Invasive aspergillosis is the most severe form of aspergillosis infection and is caused by the inhalation of Aspergillus. Common symptoms of invasive aspergillosis include fever, chest pain, cough, coughing up blood, and shortness of breath.
Over 30 million people are at risk of invasive aspergillosis each year due to use of corticosteroids, chemotherapy, or other immunosuppressive agents, and over 300,000 patients develop the disease annually1. A large prospective study found that the one-year survival rate for people who had invasive aspergillosis was 59% among solid organ transplant recipients and 25% among stem cell transplant recipients2-3. In a broad U.S. healthcare network of intensive care unit autopsy studies, aspergillosis was among the top four most common diagnoses that likely led to death4.
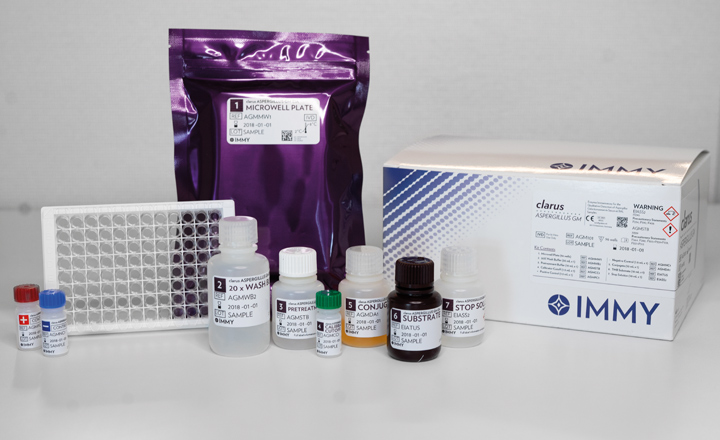
Timely diagnosis and initiation of therapy strongly influence patient outcomes.Now available from Alpha Laboratories is the clarus Aspergillus Galactomannan Enzyme Immunoassay (AGM EIA), providing results in less than three hours.
The immunoassay is sourced from IMMY, (Norman, OK), an in-vitro diagnostic manufacturer, who developed the AGM EIA, an immunoenzymatic, sandwich microplate assay used for the qualitative detection of Aspergillus galactomannan in serum and bronchoalveolar lavage (BAL) samples from patients at risk for invasive aspergillosis.
The clarus AGM EIA is a test that can be used as an aid in the diagnosis of aspergillosis when tested in conjunction with other diagnostic procedures such as microbiological culture, histological examination of biopsy samples, and radiography. The diagnostic test is intended for laboratory professional use. It provides results in less than three hours, reducing the time to proper patient care. Laboratory technicians find the diagnostic test can improve their workflow and requires fewer repeat samples. The AGM EIA has also been shown to be highly sensitive and specific.
To date, the clarus AGM EIA has been registered and approved for distribution in four key regions: Brazil (ANVISA), The European Union (CE), India (CDSCO), and The United Kingdom (MHRA).
Reduce time to patient outcome & increase workflow today with the clarus Aspergillus GM EIA. For further information, click here or contact Alpha Laboratories using the green "Request Information" button below.
References1. Invasive Aspergillosis. Leading International Fungal Education. https://www.life-worldwide.org/fungal-diseases/invasive-aspergillosis
2. Webb BJ, Ferraro JP, Rea S, Kaufusi S, Goodman BE, Spalding J. Epidemiology and clinical features of invasive fungal infection in a US health care networkexternal icon. Open Forum Infect Dis 2018 Jul 31;5(8):ofy187.
3. Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Databaseexternal icon. Clin Infect Dis. 2010 Apr 15;50(8):1091-100.
4. Webb BJ, Ferraro JP, Rea S, Kaufusi S, Goodman BE, Spalding J. Epidemiology and clinical features of invasive fungal infection in a US health care networkexternal icon. Open Forum Infect Dis 2018 Jul 31;5(8):ofy187.