Fast, accurate identification is the basis of GMP, especially with new EU Annex 1 regulations. In this article, Jessica Rayser (Product Manager) and Morgan McCarraher (Associate Product Manager) at Charles River Microbial Solutions talk to rapidmicrobiology.com on why Accugenix® Microbial Identification Service stands out from the rest, how the service is built with the customer's needs in mind and how they can help meet your regulatory requirements.
Q: Why do pharmaceutical labs need precise microbial identification?
A: Pharmaceutical manufacturing sites are required by regulatory agencies to establish comprehensive and reliable quality systems and environmental monitoring (EM) programs, which measure the state of control of their manufacturing and ensure data integrity. When bacterial or fungal isolates are recovered, it is critical to accurately identify them; as this ensures sound operational decisions, a complete tracking and trending program, and reliable risk assessments.
Tracking and trending of bioburden gives you the complete snapshot of your environment and allows you to predict potential microbial control issues and proactively prevent problems before they arise. When contaminations do occur, the reports can facilitate a full investigation of root causes and quick remediation. The identifications must be accurate to reliably assess and mitigate risk to the manufacturing process, final product, environment, operators, and – most importantly – the patient. Focus on trending EM data, in addition to the importance of species-level identification, is reinforced by regulatory guidelines such as Annex 1, which advocates the use of modern technologies.
Q: How does Charles River’s Accugenix® microbial identification portfolio meet this requirement?
A: Accurate microbial identifications are dependent on the method, how that method is executed, how the data is analyzed, and the reference library. All of Charles River’s eight global Accugenix® laboratories offer accurate microbial identifications through our AccuGENX-ID® DNA sequencing service and AccuPRO-ID® MALDI-TOF service. Our validated custom Laboratory Information Management System (LIMS) oversees all operations, ensuring data integrity and chain of custody while reducing the potential for human error. Accugenix® gives utmost priority to routinely add to, curate, and qualify its validated microbial libraries to ensure superior performance, reliability, and relevancy.
Our laboratories are cGMP-compliant and ISO 17025 accredited by the American Association for Laboratory Accreditation (A2LA). Our web portal and identification reports are 21 CFR Part 11 compliant, and we also offer complimentary Tracking and Trending. Our advanced services also include: strain typing to either determine the root cause of contamination or to confirm the identity of production strains, strain typing assay development, and sequencing of alternative gene targets for species-level identification of closely related organisms in groups such as Burkholderia cepacia complex and Bacillus cereus.
Q: What are the advantages of each identification platform and how do customers choose?
A: Charles River’s Accugenix® brand offers DNA sequencing through our AccuGENX-ID® service and MALDI-TOF identifications through our AccuPRO-ID® service. DNA sequencing is considered the ‘gold standard’ of identifications and is the most accurate and reproducible platform. It can accept a variety of sample types and preparations, whereas MALDI requires a live, fresh culture that is less than 72 hours old. DNA sequencing can provide additional information since it’s based on phylogenetics, whereas MALDI identifications are not phylogenetic.
DNA sequencing is more labor-intensive and thus more expensive. We normally recommend sequencing as the best method for identifications, but if customers have a limited budget, MALDI-TOF provides a good alternative with significantly better accuracy over phenotypic identification methods. Moreover, the criticality of samples should also be considered; critical samples should be identified by sequencing – the most accurate method – whereas routine samples could be identified by MALDI.
Q: Why is Fatty Acid Analysis not part of the offering?
A: Fatty acid analysis has been used for the identification of microorganisms after it was determined that differences in fatty acid chain length were a good marker for different species. However, sample preparation is very extensive and error-prone for this technique, and the fatty acid profiles can change if the growth conditions are not carefully controlled. Small, limited libraries also prohibited the use of this technology.
The development of DNA sequencing and MALDI-TOF mass spectrometry technologies has provided easier, faster, and more accurate identification methods.
Q: What advantages do Accugenix® microbial identification services offer over other service providers?
A: Charles River’s Accugenix® laboratories have the experience and technology necessary to rapidly and accurately handle all of your microbial identification needs, improve your processes, and reduce your costs while helping to protect your manufacturing process and brand reputation.
Because we are the industry leader in microbial identification, our scientists have built robust, validated assays and databases that contain the most comprehensive bacterial and fungal species entries found in today’s industrial production and research environments, assuring an accurate answer for virtually every sample we sequence. Our proprietary identification services have a 98% reportable rate and a 99% on-time delivery rate. Dedicated technical support, including phylogenetic and mass spectrometry experts, is available to answer all questions pertaining to result interpretation. Additionally, Accugenix® provides a secure, 21 CFR Part 11 compliant data management web portal for our customers, which simplifies sample submission and provides easy access to ID reports. All identification result data is automatically aggregated into validated tracking and trending reports for Environmental Monitoring (EM) program analysis.
With our global footprint, we can ensure a harmonized ID program for global companies, no matter where individual sites are located.
Q: What is the workflow for using the Accugenix® service?
A: All Accugenix® services can be requested through our secure online customer web portal. Customers can electronically submit samples or data files for identification. The status of submitted samples can be checked, test results downloaded, and invoices reviewed, all in one place. Once samples are submitted electronically, they can be packaged and shipped to any of our eight Accugenix® laboratories. Manuals, guides, and tutorials are available on our website that describes how to use the web portal, how to package your samples, and how to ship them to each lab.
The Accugenix® customer web portal has many features and benefits including:
- Sample management from electronic submission to reports
- Sample information available 24/7
- Real-time sample status
- Automatic notifications of shipments and reports
- Invoice management
- View, download, and print archived reports and forms
- Highest level of data encryption for security
- Client-specific usernames and password protection
- Compliance with GAMP5 standards for validation of automated systems
- Reduction of paperwork
- Improved productivity
- Elimination of redundant entries
- Customized distribution lists for reports and notifications
- A single electronic request form for genotypic and proteotypic identification services with an unlimited number of sample entries
The table below illustrates which sample preparation method can be used with the services we offer and the turnaround time (TAT) associated with the service.
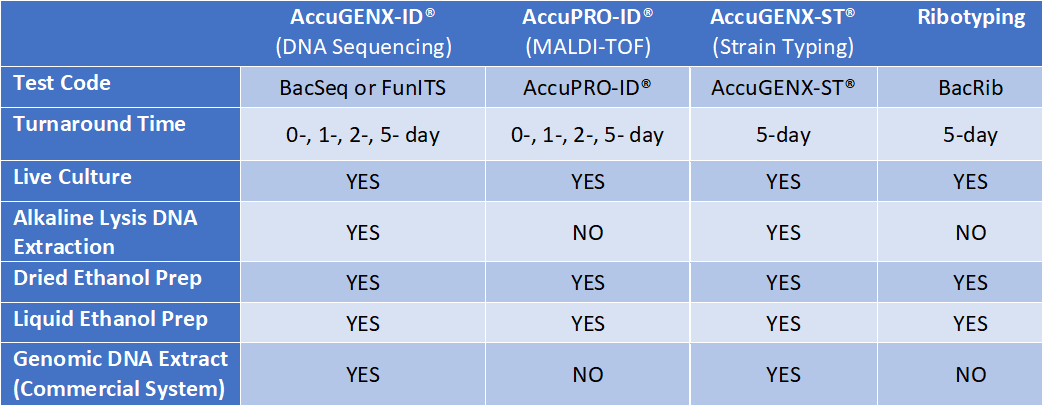
*The information listed in this table is based on Newark, DE. TAT may differ depending on the location.
Q: What are the future developments for Charles River’s microbial identification services?
A: Accugenix® is always considering how we can better meet pharmaceutical, consumer care, medical device, and other manufacturing industries’ needs! We are currently evaluating novel technologies for additional applications and services, exploring new applications of existing technologies, and investigating other development opportunities for potential business expansion. Stay tuned for exciting news!
Click here to see how the Accugenix® lab operates by taking a guided virtual tour: