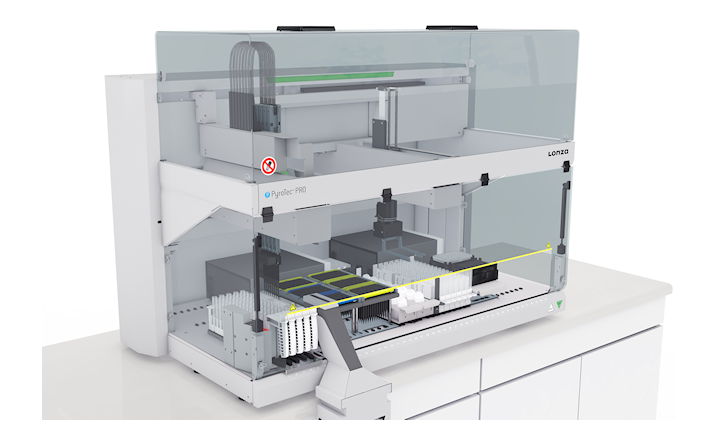
Lonza will unveil PyroTec™ PRO, the first-ever fully automated, plate-based robotic solution for endotoxin detection at SLAS2019 International Conference and Exhibition (2-6 February, Washington, DC, USA). Integrated with the latest version of Lonza’s proprietary dynamic control WinKQCL™ 6.0 Software platform, the new system has been designed to meet the needs of rapidly changing requirements of QC testing laboratories for fully automated processing of simple to complex sample matrices.
At Booth #749 Lonza’s experts will provide specialist consultation along with live product demonstrations showcasing the unique benefits of the PyroTec™ PRO Automated Solution. As a powerful combination of robotic liquid-handling technology with an automation software module, the system:
- improves data integrity organically with the capture of new metadata from the automated preparation, adding traceability into tracking, trending and audit controls
- takes any new and existing templates and dynamically “script” the instructions to an automation template with relatively minimal effort from the end user, regardless of how complex the sample type or testing requirements
- enhances assay robustness and reproducibility for increased confidence in the accuracy and precision of results
- significantly reduces manual intervention, simplifying QC testing workflows and eliminating the human error potential
- reduces re-test rates, as well as out-of-specification and out-of-trend deviations, thereby improving the laboratory’s performance
- integrates with laboratory information management systems or Lonza’s MODA™ Solution, facilitating fully paperless workflows and traceability of sample lifecycle
- offers considerable cost savings compared with conventional cartridge-based systems, which require the use of expensive reagents
- aligns with the U.S. Food and Drug Administration’s (FDA’s) Process Analytical Technology Initiative and Data Integrity requirements and is fully compliant with the U.S. Pharmacopeia Bacterial Endotoxin Test guidance
“The introduction of the PyroTec™ PRO Automated Robotic Solution and WinKQCL™ 6.0 Software marks a milestone in endotoxin detection, allowing pharmaceutical manufacturers to replace manual, error-prone processes with a fully automated solution,” said Robert Porzio, Product Manager for Endotoxin Detection at Lonza. “We look forward to demonstrating at SLAS2019 how the system can elevate endotoxin testing to a whole new level of efficiency, regardless of the complexity of sample/diluent types and analytical requirements.”
Further information can be found at Lonza’s Booth #749 at SLAS2019, 2-6 February or via www.lonza.com/endotoxin-automation