Liofilchem has received clearance from the FDA to market in the United States the MTS™ (MIC Test Strip) Penicillin G, a quantitative assay for determining the Minimum Inhibitory Concentration (MIC) of Penicillin G against Streptococcus pneumoniae, Streptococcus pyogenes (Group A), Streptococcus dysgalactiae (Group C & G), Streptococcus anginosus (Group C & G), Streptococcus constellatus (Group C & G), Streptococcus intermedius (Group C & G).
More technical details on the Penicillin G MIC Test Strip IFU
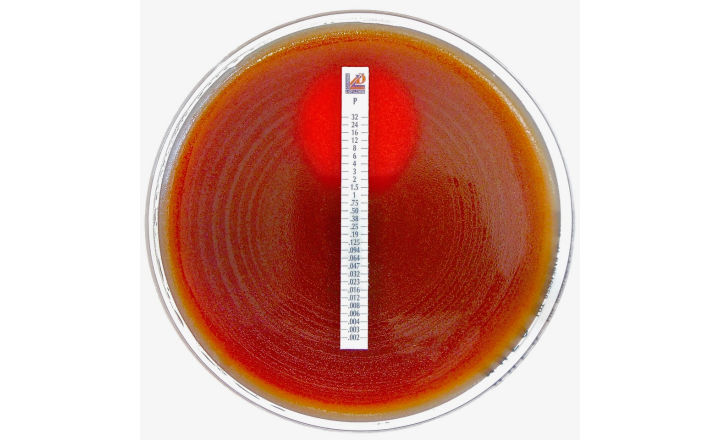
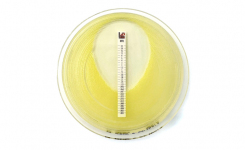
MTS™ (MIC Test Strips) are porous strips with special features (International Patent) that are impregnated with a predefined concentration gradient of antibiotic, across 15 two-fold dilutions of a conventional MIC method. On one side of the strip there is a printed MIC scale in μg/mL and a code that identifies the antimicrobial agent.
MTS™ Penicillin G 0.002-32 μg/mL
- 10 strip pack: ref. 921031
- 30 strip pack: ref. 92103
- 100 strip pack: ref. 921030
Penicillin G is the most recent FDA approved item in the MTS™ (MIC Test Strip) product range, following approvals of
- Vancomycin
- Dalbavancin
- Ceftolozane-tazobactam
- Meropenem
- Ceftazidime
- Telavancin
- Tedizolid
- Delafloxacin
- Clindamycin
- Erythromycin
- Linezolid
- Meropenem-vaborbactam
- Azithromycin
- Ceftazidime-avibactam
- Plazomicin
strips. In the meantime, the rest of the MTS™ range is available in the United States as research use only devices, while the entire MTS™ product catalog is CE marked and fully available as IVD for clinical diagnostics purposes in Europe and approved in Canada by Health Canada. MTS™ is also registered at the competent Authority in many countries outside Europe as a clinical diagnostic device.
The current MTS™ range comprises antibiotics, antifungals, antimycobacterials, and combined strips for detection of resistance mechanisms (ESBL, MBL, KPC, AmpC and GRD) for over 150 items.