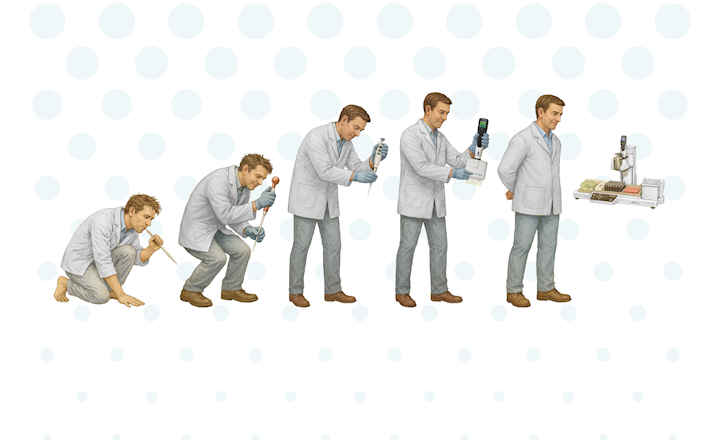
The advantages of implementing automation are no longer just for high-volume, large manufacturing organizations, as robotics represents a new paradigm for endotoxin testing, streamlining QC processes and providing widespread operational benefits across organizations of all sizes. The Nexus 200 is the next evolution of our data integrity compliant, fully enclosed robotic instrument, capable of processing up to 120 samples of undiluted or complex serial dilutions with multiple diluents for water, in-process, and final product testing via our Endosafe® LAL cartridge technology.
Traditional endotoxin testing methods have required hours of extensive assay and sample preparations, multiple dilutions, and other tasks that inherently increase the risk of contamination due to open systems and good-old-fashioned human intervention. These factors are no longer a worry in the laboratory with the help of automation and a fully enclosed environment.
Smart automation with the Endosafe Nexus 200 can improve overall process efficiency, including:
- Minimizing variation, time, and resources of performing both laboratory and out-of-specification investigations.
- Improving workflow of quality control operations, which can reduce product lead times by 60-70 percent, enabling real-time product releases.
- Avoiding the cost and time needed to train individuals to perform repetitive tasks to allocate to more high-priority tasks, such as interpreting data, analyzing trends, and proactively addressing risks.
Reducing the risk for human error in testing processes by implementing automation, leading to greater confidence in product quality and setting a standard of excellence in line with the new revisions to Annex 1. The Endosafe® Nexus 200™ uses FDA-licensed limulus amebocyte lysate (LAL) cartridges. This technology has all the necessary components included within one easy-to-use disposable cartridge, including precise amounts of LAL reagent and positive and negative control channels. They’ve also undergone a stability study which proves their sustainability over an extended period for use on longer automation runs.
The combination of this compendial cartridge technology with the innovation of the Endosafe® nexgen-MCS™ multi-cartridge system, as well as the Hamilton Microlab® Nimbus platform, makes the Nexus 200™ the latest in controlled automation of bacterial endotoxin testing.
Powered by the latest Endosafe® EndoScan-V™ software (version 6.1), the Nexus 200™ is also optimized for LIMS integration, improved traceability, security, and data management. These features allow for data integrity concerns to be a thing of the past. The addition of Charles River Cortex™ analysis software to your suite for investigative evaluation and process monitoring allows you to track and trend your endotoxin data in real time and maintain a centralized state of control.
It’s still easier than ever to receive the support you can trust with Charles River’s expert team of implementation and support specialists. Our experts are available for your convenience to ensure preventative servicing, maintenance, and repairs of the system are there for when you need it.
Fully automate your endotoxin testing